Reaction Coordinate Diagram Intermediate, Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrugzfvnwfdzjeldlont1eobsw2wdd7swzs4fcs945f3vchjrhl Usqp Cau
- 2 Reaction Coordinate Diagram Including Intermediate Structures For The Download Scientific Diagram
- Answer Label The Energy Diagram For A Two Clutch Prep
- Label The Energy Diagram For A Two Step Reaction Home Work Help Learn Cbse Forum
- File Reaction Coordinate Diagrams Showing Equilibrium Png Wikimedia Commons
- Local Clicker
- Figure 2 Free Energy Reaction Coordinate Diagram
- Reactionprofiles
- What Are The Names Of Land 2 Respectively In The Energy Diagram 2 Energy Reaction Coordinate Homeworklib
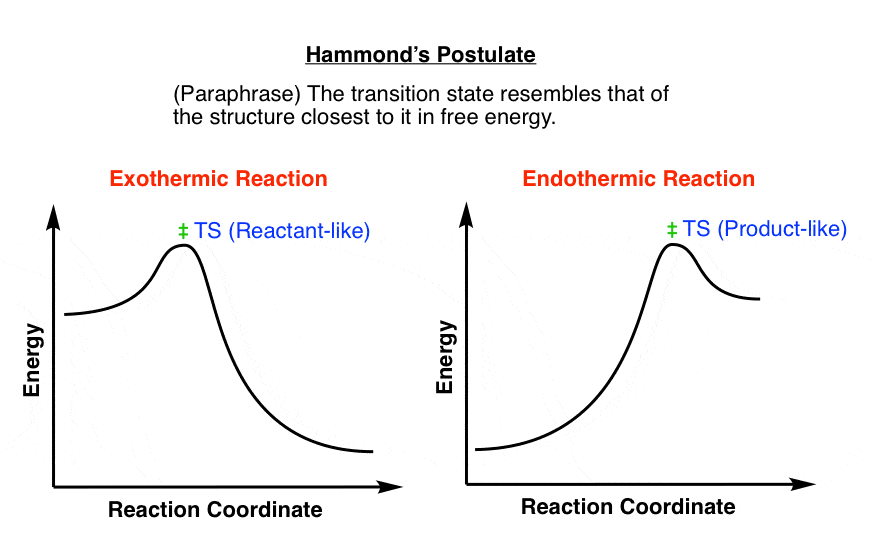
- Hammond S Postulate Master Organic Chemistry
- E1 Energy Diagram Loss Of Leaving Group Is The Rate Determining Step Energy Activities Organic Chemistry Chemistry
Find, Read, And Discover Reaction Coordinate Diagram Intermediate, Such Us:
- Answered Draw A Reaction Coordinate Diagram For Bartleby
- Ch 8 Sn1 Mechanism
- Which Type Of Reaction Does This Diagram Represent Free Wiring Diagram
- What Is The Difference Between A Transition State And An Intermediate Organic Chemistry Tutor
- Solved Mapoob The Letters On The Left Of The Reaction Coo Chegg Com
If you re looking for Lv76610 6c Circuit Diagram you've arrived at the ideal place. We ve got 104 images about lv76610 6c circuit diagram adding pictures, photos, photographs, backgrounds, and more. In these page, we additionally provide variety of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.

Diagram Bowen Reaction Diagram Full Version Hd Quality Reaction Diagram Fauxwiring2k Atuttasosta It Lv76610 6c Circuit Diagram
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gctnbxa2h Eq2ppxbypdlvlnzsrgyww Hudtr1icor2aiftsw164 Usqp Cau Lv76610 6c Circuit Diagram
In an energy diagram the vertical axis represents the overall energy of the reactants while the horizontal axis is the reaction coordinate tracing from left to right the progress of the reaction from starting compounds to final products.
Lv76610 6c circuit diagram. The arrow marked in the question represents the activation energy which is the energy barrier that must be overcome in order for the reactants to form products. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. This is an exothermic reactionheat is given off and should be favorable from an energy standpoint.
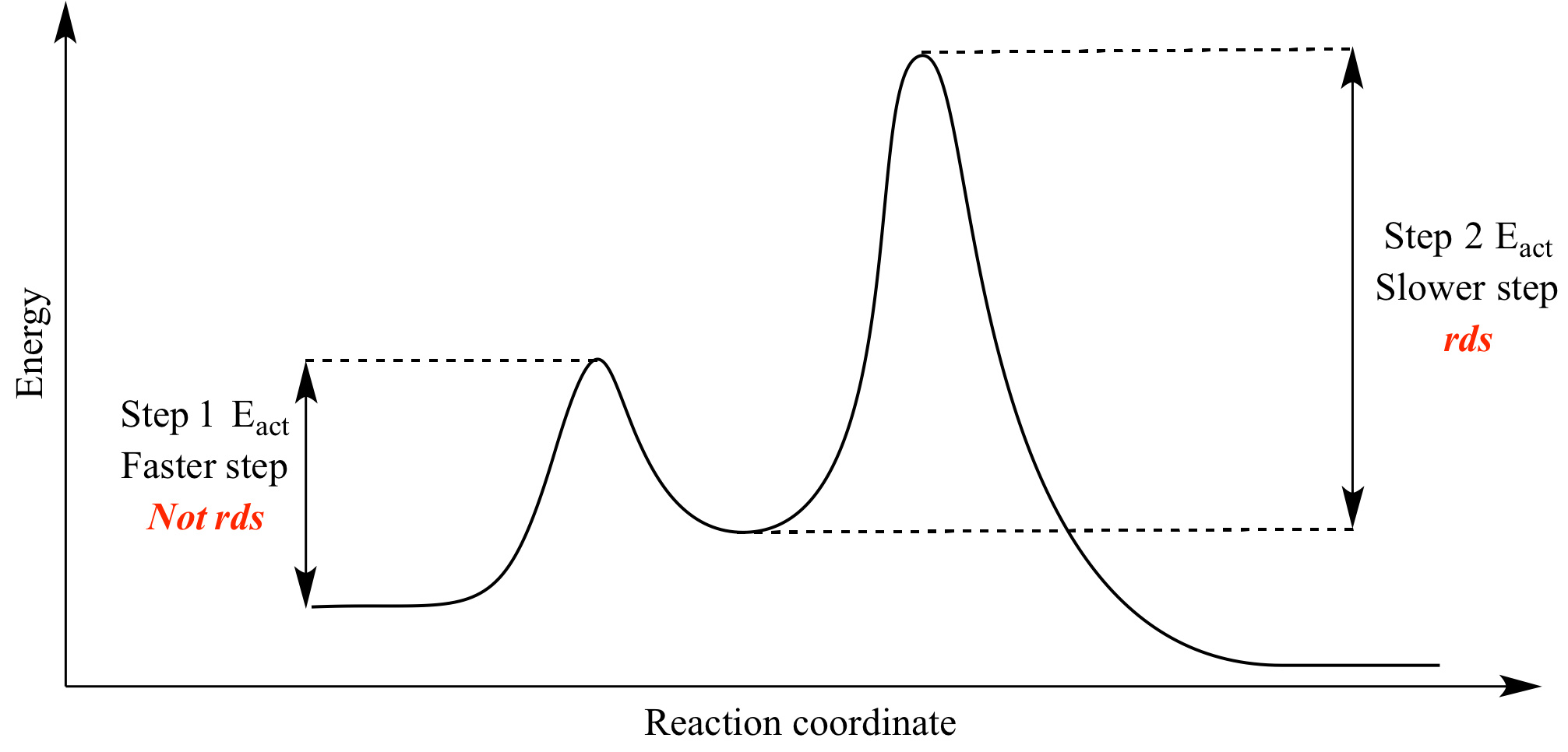
Ron rusay a reaction coordinate energy diagram thermodynamic quantities. In this example b is at a lower total energy than a. The reaction involves two steps step 1 is the slowest step and step 2 is the fastest step.
The reaction coordinate plotted along the abscissa represents the changes in atomic coordinates as the system progresses from reactants to productsin the very simplest elementary reactions it might correspond to the stretching or twisting of a particular bond and be shown to a scale. Given the following reaction sketch a reaction coordinate graph. Starting materials nitronium ion box 2.
The rate is mathematically represented by a rate constant k. Intermediate final product no 2 free response reaction coordinate diagram e one. Geometrysteric effects of reactants and intermediates 6.
The reaction is a reaction between hydrogen gas and brown vapor of iodine monochloride. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction the reaction for the transition states and intermediate states.
Coordinate in terms of activation energies in the forward and reverse direction and based on activation energies the rate at which the reaction occurs in the forward and reverse directions. If the barrier energy for going from intermediate to product is much higher than the one for reactant to intermediate transition it can be safely concluded that a complete equilibrium is established between the reactant and intermediate. The reaction above has three steps three barriers and two intermediates.
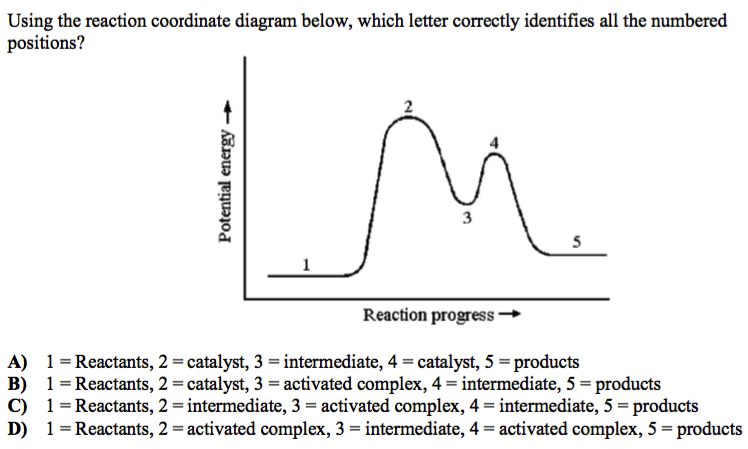
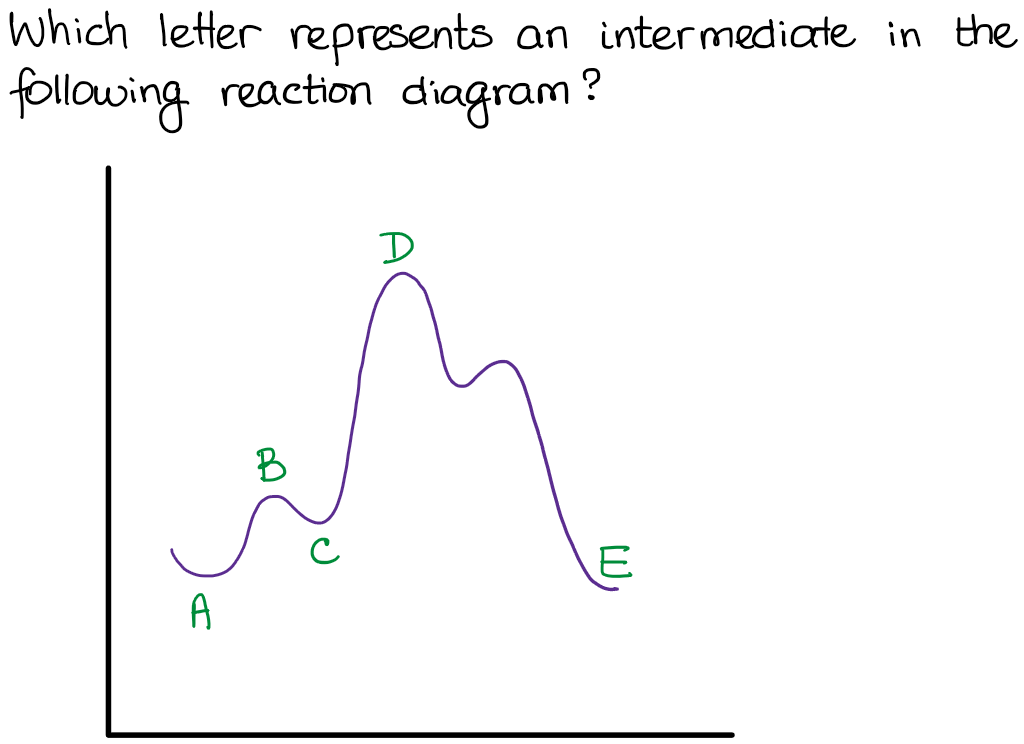
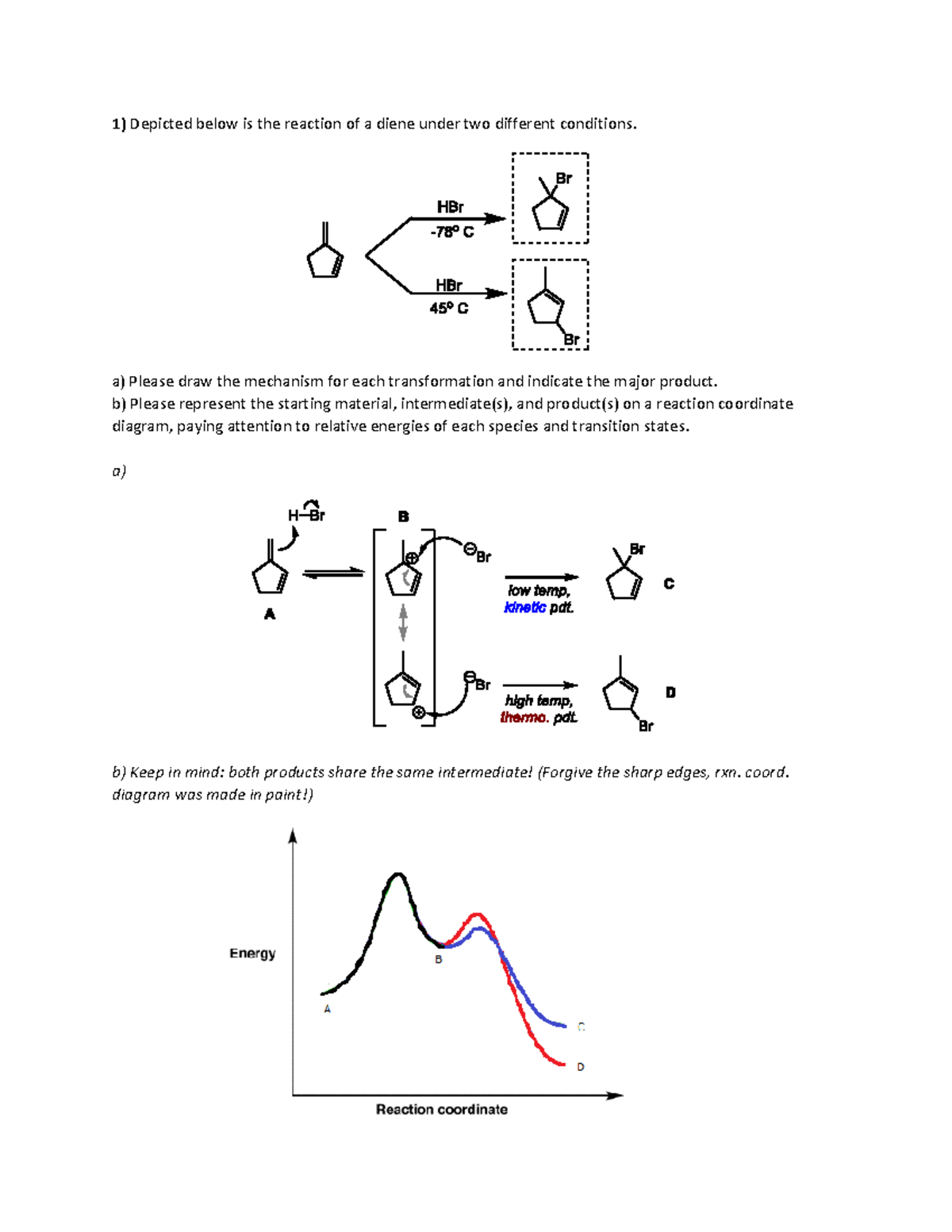
How will this change affect the rate of the reaction. This question is in reference to drawing reading and understanding a reaction. Any minima that exist between the reactants and the products along the reaction coordinate are intermediates.
4 sentences max hno 3 h 2 so 4 no 2 box 1. The diagram below is called a reaction coordinate diagram. Reaction coordinate diagrams also give information about the equilibrium between a reactant or a product and an intermediate.
Catalysts thermodynamics kinetics. On the far left of the diagram are the reactant species and on the far right are the product species. How will the reaction coordinate diagram change if benzene is replaced by methylbenzene.
Lv76610 6c Circuit Diagram, Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrugzfvnwfdzjeldlont1eobsw2wdd7swzs4fcs945f3vchjrhl Usqp Cau
- Which Step Below Is Rate Determining Chemistry Stack Exchange
- Solved Draw Reaction Coordinate Diagrams For The Following Rea Chegg Com
- Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
Lv76610 6c Circuit Diagram, Which Step Below Is Rate Determining Chemistry Stack Exchange
- Hammond S Postulate Master Organic Chemistry
- Ppt Energy Reaction Coordinate Diagrams Thermodynamics Kinetics Powerpoint Presentation Id 1586358
- Enzyme Catalysis Intermediates Along The Reaction Coordinate
Lv76610 6c Circuit Diagram, Biol 402 Hw 4 Flashcards Quizlet
- Solved The Letters On The Left Of The Reaction Coordinate Chegg Com
- Reactionprofiles
- Solved 2 Match The Potential Energy Diagram Reaction Coordinate Diagram With The Reaction A Correctly Identifying The Reactions With The Pe Rc D Course Hero
More From Lv76610 6c Circuit Diagram
- 1997 F150 Fuse Diagram
- What Is A Fishbone Diagram In Healthcare
- What Does Complement Mean In Maths Venn Diagrams
- 2010 Honda Civic Fuse Box Diagram
- Dip Switch Schematic
Incoming Search Terms:
- 5 6 Reaction Energy Diagrams And Transition States Chemistry Libretexts Dip Switch Schematic,
- Hammond S Postulate Master Organic Chemistry Dip Switch Schematic,
- Enzyme Catalysis Intermediates Along The Reaction Coordinate Dip Switch Schematic,
- Which Is The Correct Reaction Coordinate Diagram For The Following Dip Switch Schematic,
- Ppt Energy Reaction Coordinate Diagrams Thermodynamics Kinetics Powerpoint Presentation Id 1586358 Dip Switch Schematic,
- Reaction Energy Concepts Dip Switch Schematic,