Molecular Orbital Theory Equation, Https Thinfilmsliterature Files Wordpress Com 2017 11 10 5 Molecular Orbital Theory Pdf
- Molecular Orbital Theory Chemistry Video Clutch Prep
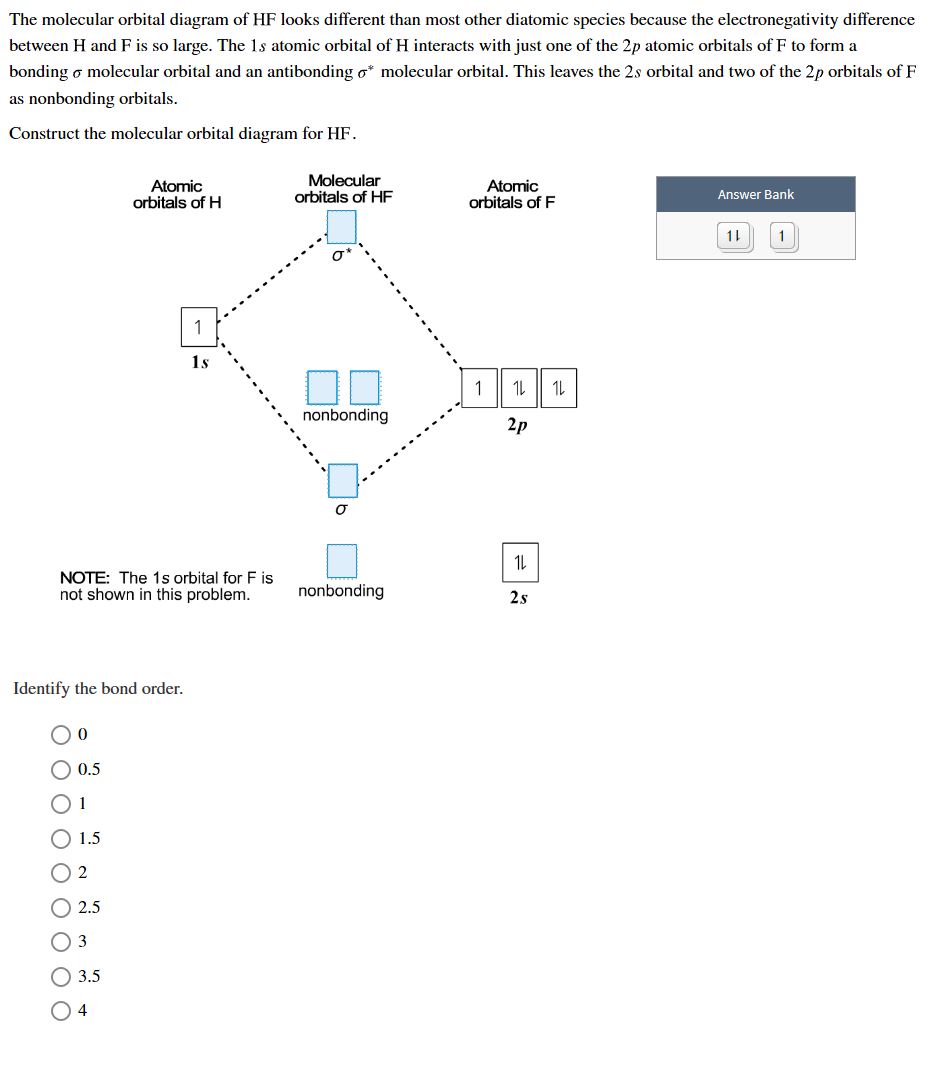
- Answered The Molecular Orbital Diagram Of Hf Bartleby
- Delocalized Bonding And Molecular Orbitals
- Molecular Orbital Theory Lcao Dry Lab Chemistry Libretexts
- Molecular Orbitals Introductory Chemistry 1st Canadian Edition
- 2
- Molecular Orbitals Introductory Chemistry 1st Canadian Edition
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcqny 3 Pa3 Xvpunc1evhr2fd9v2vr7xzxotxl4ppzcmbaqa3hx Usqp Cau
- Chemistry Molecular Structure 43 Of 45 Molecular Orbital Theory Bond Order 2 Li Youtube
- Introduction To Molecular Orbital Theory
Find, Read, And Discover Molecular Orbital Theory Equation, Such Us:
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcqny 3 Pa3 Xvpunc1evhr2fd9v2vr7xzxotxl4ppzcmbaqa3hx Usqp Cau
- Compchem 03 03 Foundations Of Molecular Orbital Theory Huckel Theory For The Allyl System Youtube
- Molecular Orbital Theory Boundless Chemistry
- Molecular Orbital Theory Pdf Archive
- Molecules
If you are searching for Stun Gun Schematic you've come to the ideal location. We have 104 graphics about stun gun schematic adding pictures, photos, pictures, wallpapers, and much more. In such web page, we additionally have variety of graphics out there. Such as png, jpg, animated gifs, pic art, logo, black and white, transparent, etc.
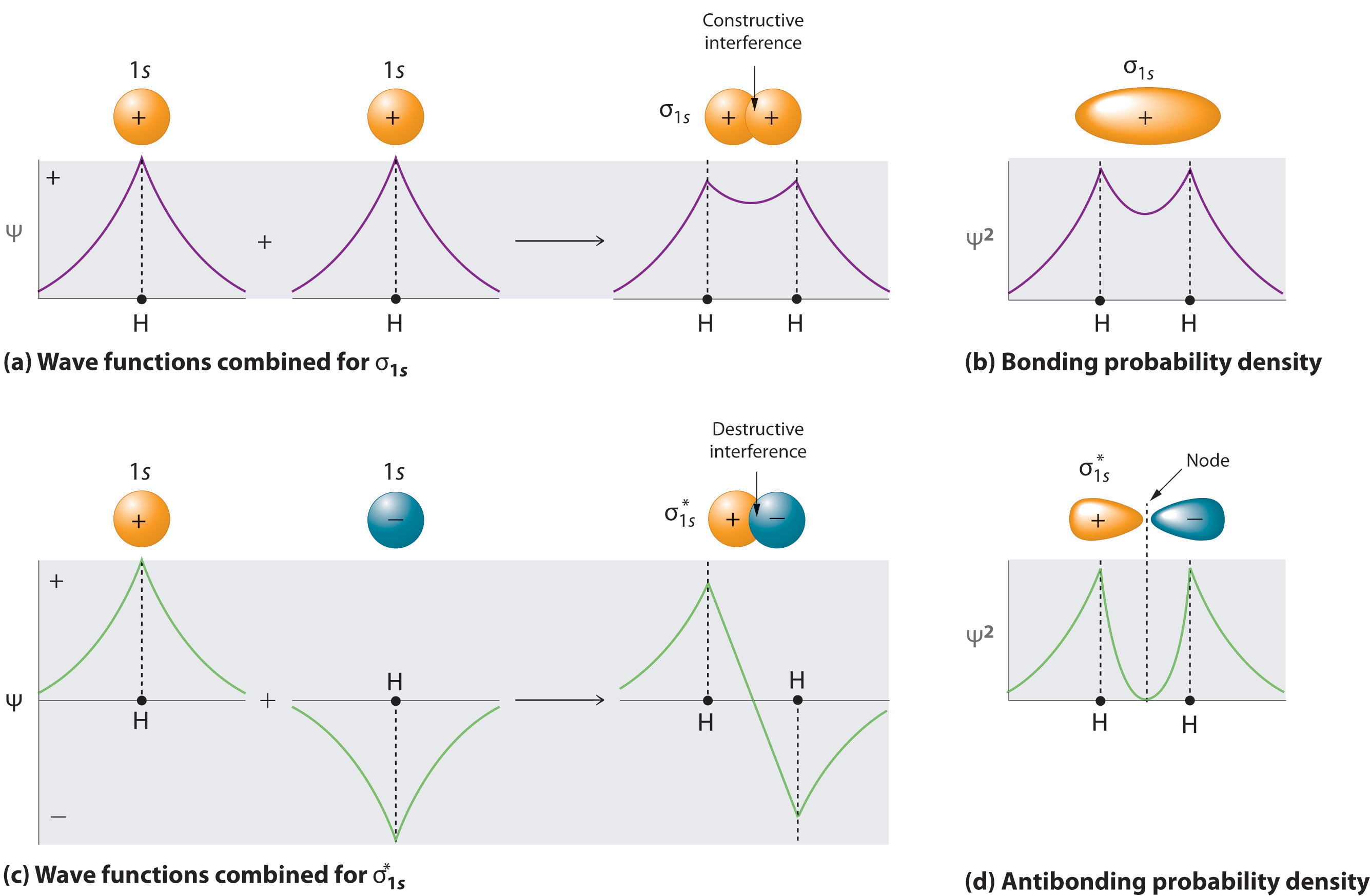
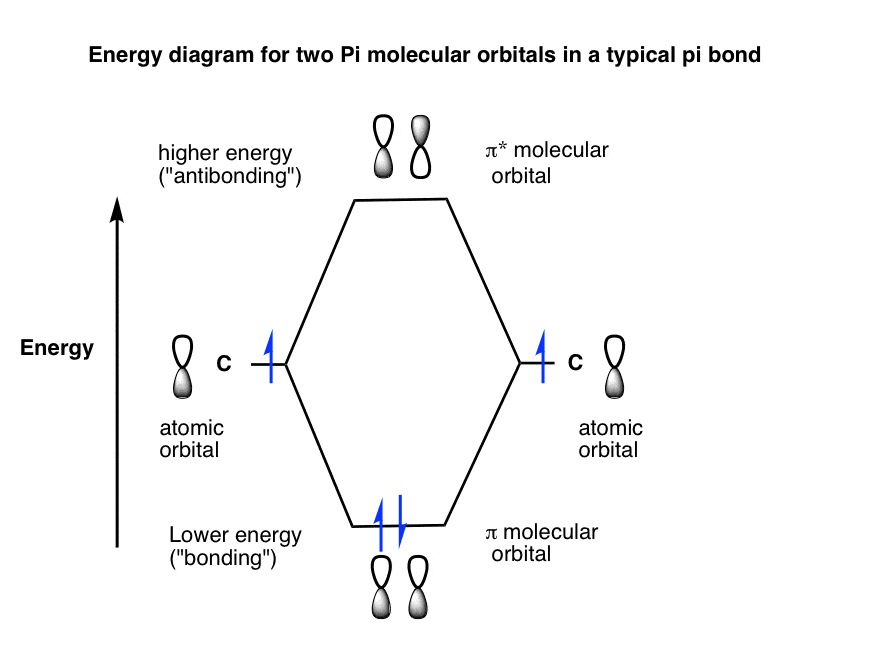
The corresponding anti bonding molecular orbitals are represented by s p d.

Stun gun schematic. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atoms nucleusthe term atomic orbital may also refer to the physical region or space where the electron can be. This electron configuration gives a bond order of 2 1212. We can represent them as ps mo ps a ps b.
It was proposed early in the 20th century. In molecular orbital theory electrons in a molecule are not assigned to individual chemical bonds between atoms but are treated as moving under the influence of the atomic nuclei in the whole molecule. Mathematically molecular orbitals are an approximate solution to the schrodinger equation for the electrons in the field of the molecules atomic nuclei.
In atomic theory and quantum mechanics an atomic orbital is a mathematical function describing the location and wave like behavior of an electron in an atom. When molecular orbital forms by the subtraction of wave function the type of molecular orbitals formed are antibonding molecular orbitals. The lowering of the energy of bonding molecular orbital than the combining atomic orbital is called stabilization energy and similarly increase in energy of the anti bonding molecular orbitals is called destabilization energy.
This equation is substituted in the schroedinger equation. Molecular orbitals are obtained by combining the atomic orbitals on the atoms in the molecule. They have higher energy than atomic orbitals.
One of the molecular orbitals in this molecule is constructed by adding the mathematical functions for the two 1 s atomic orbitals that come together to form this molecule. They are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule or other molecular orbitals from groups of atoms. Mathematically molecular orbital theorists describe the process by the equation psi 1sa 1sb where psi psi stands for the molecular orbital while 1sa and 1sb represent the atomic orbitals for h a and h b respectively.
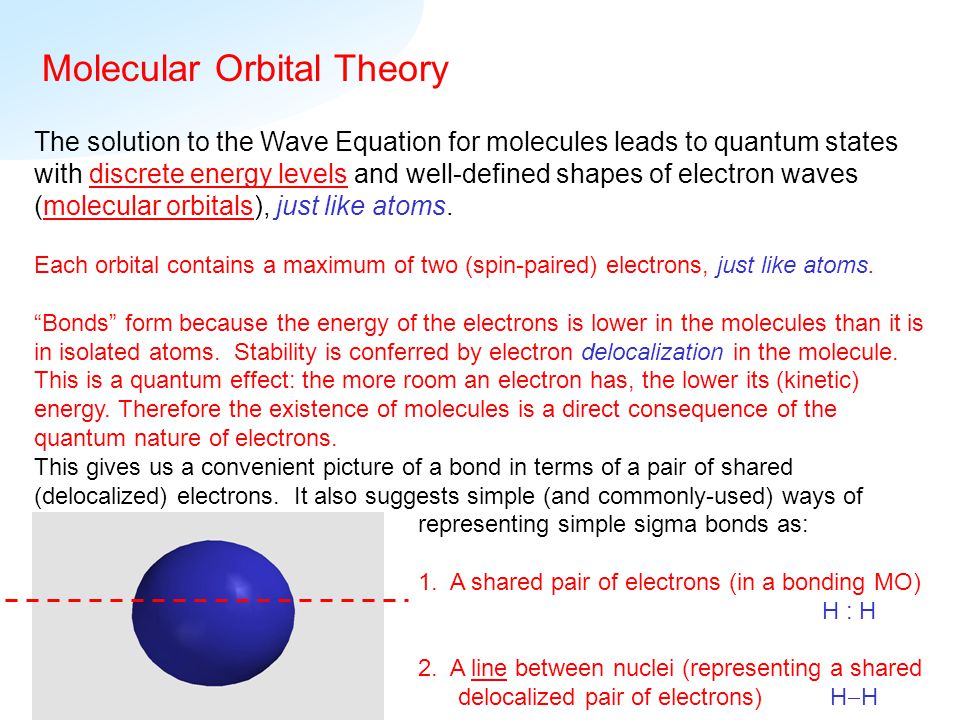
In chemistry molecular orbital theory is a method for describing the electronic structure of molecules using quantum mechanics. Quantum mechanics describes the spatial and energetic properties of electrons as molecular orbitals that surround two or mor. Jens martensson molecular orbital theory the molecular orbital theory often abbreviated to mot is a theory on chemical bonding developed at the beginning of the twentieth century by f.
Hath psii rangle ei psii rangle nonumber with hath the hamiltonian and ei the energy corresponding to the molecular orbital to give. Because the first two electrons completely fill the s 1s molecular orbital the pauli principle states that the third electron must be in the s 1 s antibonding orbital giving a s 1 s 2 s 1 s 1 electron configuration. Mulliken to describe the structure and properties of different molecules.
Stun Gun Schematic, Molecular Orbital Theory Pdf Archive
- Molecular Orbital Theory Mot Chemistry Study Material Emedicalprep Com Emedicalprep
- Introduction To Molecular Orbital Theory
- Supplementary Course Topic 4 Ppt Download
Stun Gun Schematic, 4 Molecular Structure The Schrodinger Equation For Molecules The Born Oppenheimer Approximation 4 1 Molecular Orbital Theory The Hydrogen Ppt Download
- Introduction To Molecular Orbital Theory
- Mo Diagrams
- 9 7 Molecular Orbitals Chemistry Libretexts
Stun Gun Schematic, Pictorial Molecular Orbital Theory Chemistry Libretexts
- Molecular Orbital Theory Mot Inorganic Chemistry Chemistry Funda
- Introduction To Molecular Orbital Theory
- Filling Of Molecular Orbital And Bond Order Molecular Order Bond Order Theory Of Bonding Youtube
More From Stun Gun Schematic
- Ldr Schematic
- Probability Tree Diagram Pdf
- Diagram Of Transpiration In Plants
- Basic Relay Diagram
- 2000 Nissan Frontier Fuse Box Diagram
Incoming Search Terms:
- Molecular Orbital Concepts 2000 Nissan Frontier Fuse Box Diagram,
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gctupcydqvicwfxsm0bhskdih7yg1gacbhyqh Lrep4llz53vwfw Usqp Cau 2000 Nissan Frontier Fuse Box Diagram,
- Compchem 03 03 Foundations Of Molecular Orbital Theory Huckel Theory For The Allyl System Youtube 2000 Nissan Frontier Fuse Box Diagram,
- Density Functional Theory Hartree Fock Useful 2000 Nissan Frontier Fuse Box Diagram,
- A Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic B 2 2 B2 C 2 2 B 2 2 And N 2 2 B Draw The Lewis Structures And Molecular Orbital Diagrams For 2000 Nissan Frontier Fuse Box Diagram,
- Molecular Orbital And Valence Bond Theory 2000 Nissan Frontier Fuse Box Diagram,