O2 Molecular Orbital Diagram Bond Order, Solved The Molecular Orbital Energy Level Diagrams For 02 Chegg Com
- Bonding In Some Homonuclear Diatomic Molecules Emedicalprep
- Mo Diagram O2 O2 2 O2 O2 2 Preparation Of Gate Csir Net Uset Set Exam Youtube
- What Is The Bond Order Of Oxygen Quora
- Molecular Orbital Diagram Of O2 F2 And Ne2 Molecules Youtube
- Https Thinfilmsliterature Files Wordpress Com 2017 11 10 5 Molecular Orbital Theory Pdf
- Https Thinfilmsliterature Files Wordpress Com 2017 11 10 5 Molecular Orbital Theory Pdf
- Solved Question 2 1 Pts Use The Molecular Orbital Diagram Chegg Com
- Bond Order Ofo2 O2 O2 And O22 Is In Order A O2 Langle Class 11 Chemistry Cbse
- Molecular Orbital Energy Level Diagrams Hydrogen Hypothetical Nitrogen Oxygen
- Site Map
Find, Read, And Discover O2 Molecular Orbital Diagram Bond Order, Such Us:
- Give The Molecular Orbital Energy Diagram Of N2 And O2 Write The Bond Order Of N2 And O2 Sarthaks Econnect Largest Online Education Community
- Give The Molecular Orbital Energy Diagram Of N2 And O2 Calculate Their Respective Bond Order Write The Brainly In
- Molecular Geometry And Bonding Theories Ppt Video Online Download
- Http Butane Chem Uiuc Edu Pshapley Genchem2 A5 Book Pdf
- Use A Molecular Orbital Diagram To Determi Clutch Prep
If you are looking for And Gate Diagram you've reached the ideal place. We have 104 graphics about and gate diagram adding pictures, pictures, photos, wallpapers, and much more. In such webpage, we also have number of images available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.

Oneclass Draw Molecular Orbital Diagram And Determine The Bond Order And The Number Of Unpaired Elec And Gate Diagram
Here n b 8.
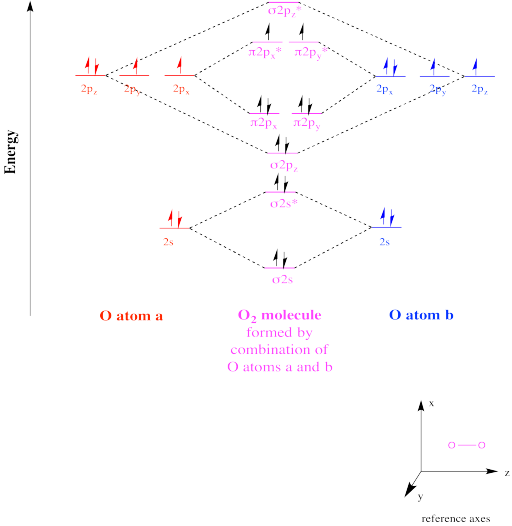
And gate diagram. For the bond order of n2 is 25 which is nitrogen ion. The electron configuration of the oxygen molecule must accommodate 16 electrons. The bond length in the oxygen species o2 o2 o2 o22 can be explained by the positions of the electrons in molecular orbital theory.
Electronic structure of oxygen atom is leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule represented as kk the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown. This ion is formed by the addition of one electrono2 e o2this additional electron will be added up in the molecular orbitalelectronic configurationbond orderhere nb 8. Therefore it is paramagnetico2 ion this ion is.
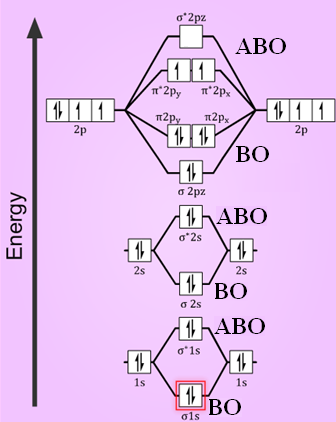
Bond order for no order by bond length. Na 5stability. The molecular orbital surrounds two or more nuclei of the bonded atoms.
Among them having lower energy is called bonding molecular orbital while the other having higher energy is called an anti bonding molecular orbital. O2 is well known to be paramagnetic and it is one of the successes of molecular orbital theory. Well the mo diagram for o2 is.
It has one unpaired electron in the molecular orbital. You can see that co is not as it has zero unpaired electrons but no is it has one unpaired electron. As the bond order is positive it is quite stablemagnetic character.
The bond order is already calculated in the. N a 4 the two oxygen atoms in a molecule of oxygen are united through two covalent. See link 1 for an energy level diagram of the bonding and antibonding orbitals in the molecule and molecular ions of oxygen.
Why o2 molecule is paramagnetic while n2 is diamagnetic. Molecular orbital diagram for oxygen gas o2fill from the bottom up with 12 electrons totalbonding order is 2 and it is paramagneticsigma2s2sigma2s. 2 atomic orbitals after overlapping form 2 molecular orbitals which differ in energy.
No no no is co a lewis acid.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrz6dgwcv Xjf769bh2f1pvjhaoq0qphrfvi5gbszseu Gxhfeu Usqp Cau And Gate Diagram
And Gate Diagram, Diatomic Species Mo Theory Chemogenesis
- Molecular Structure Atomic Orbitals
- What Is The Bond Order Of Hf Study Com
- Molecular Orbital Theory
And Gate Diagram, Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com
- Solved The Molecular Orbital Energy Level Diagrams For 02 Chegg Com
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gctupcydqvicwfxsm0bhskdih7yg1gacbhyqh Lrep4llz53vwfw Usqp Cau
- Chemistry Advanced Theories Of Covalent Bonding Chapter 8 Ppt Download
And Gate Diagram, Mo Diagram O2 O2 2 O2 O2 2 Preparation Of Gate Csir Net Uset Set Exam Youtube
- Molecular Orbitals For Carbon Monoxide
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcqfg1rd847fmpn8p8j6voqfivtc6 Uexiulpifp66ibbtpt4iqu Usqp Cau
- A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate Chemistry Students
More From And Gate Diagram
- 2010 Buick Lacrosse Fuse Box Diagram
- Plug Diagram Uk
- Lewis Dot Symbol Definition
- Process Chart Pmp
- Oxygen Bohr Diagram
Incoming Search Terms:
- Draw The Mo Diagram For O2 And Identify The Following Bon Clutch Prep Oxygen Bohr Diagram,
- Molecular Orbitals For Peroxide Ion Oxygen Bohr Diagram,
- Oneclass Draw Molecular Orbital Diagram And Determine The Bond Order And The Number Of Unpaired Elec Oxygen Bohr Diagram,
- Compare The Stabilities Of O2 O2 O22 Chemical Bonding And Molecular Structure Chemistry Class 11 Oxygen Bohr Diagram,
- Diatomic Species Mo Theory Chemogenesis Oxygen Bohr Diagram,
- Give The Molecular Orbital Energy Diagram Of A N2 And B O2 Calculate The Respective Bond Order Write The Magnetic Nature Of N2 And O2 Molecules Oxygen Bohr Diagram,