Energy Level Diagram For Oxygen, Partial Energy Level Diagram For Atomic Oxygen Transitions Download Scientific Diagram
- Hund Rule Of Maximum Multiplicity Chemistrygod
- Molecular Orbital Diagram Wikipedia
- Energy Profile Diagrams Endothermic Exothermic Reactions 3 Exothermic Reaction Chemical Energy Energy Level
- 5 19 Hund S Rule And Orbital Filling Diagrams Chemistry Libretexts
- Figure 3 From Semiempirical Scf Ci Modeling Of Ce 3 4f And 5d Energy Levels In Oxygen Doped Srs Ce Semantic Scholar
- A Draw The Energy Diagram For H2 Be2 N2 O2 B What Is Meant By The Term Bond Order Chemistry 8057743 Meritnation Com
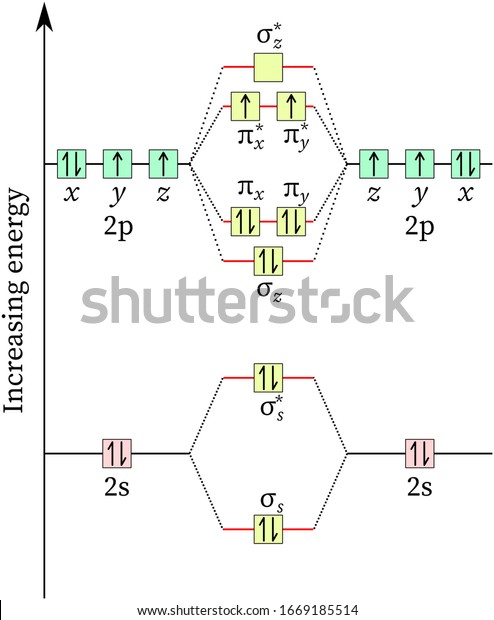
- Oxygen Molecular Orbital Diagram Energy Level Stock Illustration 1669185514
- How To Represent Electrons In An Energy Level Diagram Dummies
- Molecular Orbital Energy Level Diagrams Hydrogen Hypothetical Nitrogen Oxygen
- Http Www Mountgrace Org Uk Force Download Cfm Id 2553
Find, Read, And Discover Energy Level Diagram For Oxygen, Such Us:
- Energy Level Diagrams A 20
- How Do I Write The Electron Configuration Of An Oxygen Molecule Socratic
- Draw Molecular Energy Level Diagram Of O2 Molecule Calculate Its Bond Order Brainly In
- Tang 02 Wave Quantum Mechanic Model
- Section 5 Observation 3 Ionization Energies Of Diatomic Molecule Chemistry Libretexts
If you re looking for Making A Venn Diagram you've come to the ideal location. We ve got 104 images about making a venn diagram including pictures, pictures, photos, backgrounds, and more. In such page, we additionally provide number of images out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.

Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11 Making A Venn Diagram
You look on the periodic table and find that oxygen is atomic number 8.

Making a venn diagram. Molecular orbital diagrams are diagrams of molecular orbital mo energy levels shown as short horizontal lines in the center flanked by constituent atomic orbital ao energy levels for comparison with the energy levels increasing from the bottom to the top. Energy level diagram of oxygen molecule. This number means that oxygen has 8 protons in its nucleus and 8 electrons.
Below is a blank energy level diagram which helps you depict electrons for any specific atom. 4s has lower energy when compared to 3d. To obtain the molecular orbital energy level diagram for o 2 we need to place 12 valence electrons 6 from each o atom in the energy level diagram shown in figure 9101.
2s 2 2p 3 4 s03s. 2s 2 2p 3 4 s03s. Lines often dashed diagonal lines connect mo levels with their constituent ao levels.
Bond order prev question next question related questions 0 votes. The three dashes in 2p subshells represent the same energy. So you put 8 electrons into your energy level diagram.
S1s s1s s2s s2s p2px p2py s2pz p2px p2py p 2pz relationship between electronic configuration and molecular behaviour 1 stability of molecules in terms of bonding and antibonding electrons number. More molecular orbital diagrams for 02 are provided below. Suppose you want to draw the energy level diagram of oxygen.
You can represent electrons as arrows. Energy level diagram for molecular orbitals the first ten molecular orbitals may be arranged in order of energy as follow. Show transcribed image text complete this valence molecular orbital diagram for oxygen o2.
In this screencast andrew burrows walks you through how to construct the mo energy level diagram for o2 in order to explain its paramagnetism. Make your diagram based on the configuration. Are used to model energy.
2s 2 2p 4. 2s 2 2p 4. Draw the mo diagram for oxygen molecule and calculate its bond order and show that o2 is paramagnetic.
2s 2 2p 4. At energy level 2 there are both s and p orbitals. Therefore the order of energy level is as follows.
2s 2 2p 3 4 s03p. The bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory.
Making A Venn Diagram, Http Www Mountgrace Org Uk Force Download Cfm Id 2553
- Chem 2303 Supplementary Problems
- Draw The Molecular Orbital Diagram Of O2 Or N2 Brainly In
- Organization Of Electrons In Atoms Introductory Chemistry 1st Canadian Edition
Making A Venn Diagram, Molecular Orbital Theory
- File Oxygen Energy Level Diagram Fr Jpg Wikimedia Commons
- Energy Level Diagram For O Iii 2p 2 And S Ii 3p 3 Ions The Download Scientific Diagram
- Oxygen Molecular Orbital Diagram Energy Level Stock Illustration 1669185514
Making A Venn Diagram, Lecture 2 Page 3 Light Matter The Sun
- Draw Energy Level Diagram To Show That N2 Has Triple Bond O2 Has Double Bond F2 Has Single Bond And Chemistry Organic Chemistry Some Basic Principles And Techniques 1691566 Meritnation Com
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcskzavdbt0mkkb8ajskm0c94zo0m4zkbxub Wddsmkl Wa96t2n Usqp Cau
- How To Represent Electrons In An Energy Level Diagram Dummies
More From Making A Venn Diagram
- Converter Circuit Diagram
- 2003 Toyota Corolla Fuse Box Diagram
- Molecular Orbital Diagram Of Cof6 3
- Tree Branch Diagram
- Transformer Circuit Symbol
Incoming Search Terms:
- Why Energy Level Diagram Of Oxygen Is Different From Nitrogen Transformer Circuit Symbol,
- Molecular Orbital Energy Level Diagrams Hydrogen Hypothetical Nitrogen Oxygen Transformer Circuit Symbol,
- Solved 63 Choose The Correct Energy Level Diagram For Th Chegg Com Transformer Circuit Symbol,
- How To Represent Electrons In An Energy Level Diagram Dummies Transformer Circuit Symbol,
- Introduction To Molecular Orbital Theory Transformer Circuit Symbol,
- Molecular Orbital Diagram Wikipedia Transformer Circuit Symbol,