Molecular Orbital Diagram Of Cof6 3, Chapter 20 Transition Metals And Coordination Chemistry Ppt Download
- 21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2
- Molecular Orbital Diagram Of Cof 6 3 Complex With Six P Donor Ligands Download Table
- Https Www Lnmuacin In Epathshala Che522 Pdf
- Http Wwwchem Uwimona Edu Jm Courses C21jnotes05 Pdf
- 19 3 Spectroscopic And Magnetic Properties Of Coordination Compounds Chemistry
- Understanding The Absorption Electronic Spectra Of Coordination Compounds At Greater Depth Ligand Field Theory Chapter Ppt Download
- How To Show That Co Cn 6 3 A Yellow Complex Has A Larger Delta O Than Cof 6 3 A Blue Complex Using Knowledge Of Sigma Donor Pi Donor And Pi Acceptor Behavior And Spin Only Magnetic Moment
- Inorganic Chemistry Pages 651 700 Flip Pdf Download Fliphtml5
- Http Www Zysman Colman Com Courses Ch3514 Ch3514 20 20physical 20inorganic 20chemistry Pdf
- Https Sc Nahrainuniv Edu Iq Lectures 9226 D8 A7 D9 84 D9 81 D8 B5 D9 84 20 D8 A7 D9 84 D8 A7 D9 88 D9 84 20 D9 86 D8 B8 D8 B1 D9 8a D8 A7 D8 Aa 20 D8 A7 D9 84 D9 83 D9 8a D9 85 D9 8a D8 A7 D8 A1 D8 A7 D9 84 D8 Aa D9 86 D8 A7 D8 B3 D9 82 D9 8a D8 A9 D8 A7 D9 85 20 D9 81 D8 B1 D8 Ad 20 D9 85 D8 A4 D9 8a D8 Af 20 D8 A7 D8 A8 D8 B1 D8 A7 D9 87 D9 8a D9 85 20 D9 83 D9 84 D9 8a D8 A9 20 D8 A7 D9 84 D8 B9 D9 84 D9 88 D9 85 20 D9 82 D8 B3 D9 85 20 D8 A7 D9 84 D9 83 D9 8a D9 85 D9 8a D8 A7 D8 A1 Pdf
Find, Read, And Discover Molecular Orbital Diagram Of Cof6 3, Such Us:
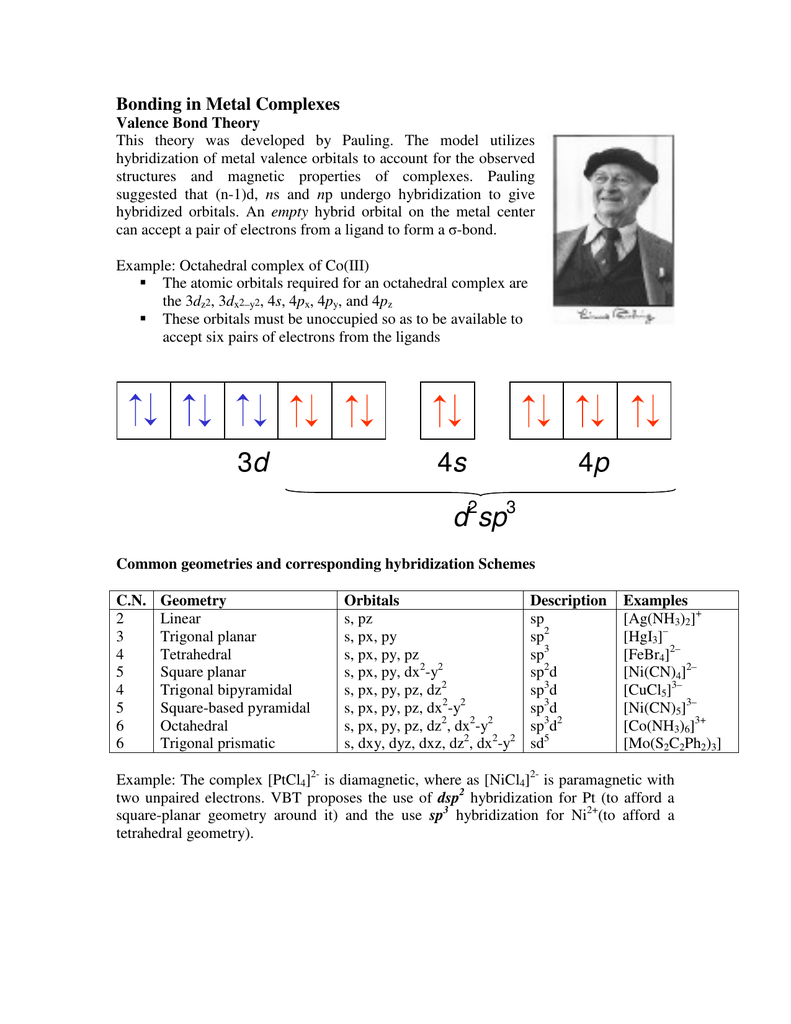
- On The Basis Of Valence Bond Theory Explain The Oxidation State Hybridisation Geometry And Magnetic Nature Of Metal In Complex Cof6 3
- Http Mcgrady Chem Ox Ac Uk Lectures Handout 202019 Pdf
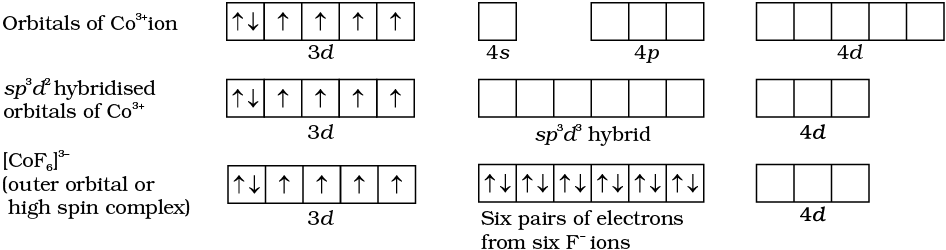
- Cof6 3 Is A Coordination Complex Ion I What Is The Oxidation Number Of Cobalt In The Complex Ii How Many Unpaired Electrons Are There In The Complex Iii State The Magnetic Behaviour
- Https Acikders Ankara Edu Tr Mod Resource View Php Id 117175
- Spectroscopic And Magnetic Properties Of Coordination Compounds Chemistry For Majors
If you are looking for Remote Circuit Diagram you've arrived at the perfect location. We have 104 graphics about remote circuit diagram adding pictures, pictures, photos, wallpapers, and much more. In these web page, we also have number of graphics available. Such as png, jpg, animated gifs, pic art, symbol, black and white, transparent, etc.
Point group oh 2.
Remote circuit diagram. Predict the number of unpaired electrons in cof63. Coordination compound coordination compound ligand field and molecular orbital theories. And show why it is a n acceptor ligand.
Log in join now 1. The six ligands can interact with the metal in a sigma or pi fashion. Since 1950 it has been apparent that a more complete theory which incorporates contributions from both ionic and covalent bonding is necessary to give an adequate account of the properties of coordination compounds.
2 draw the molecular orbital diagram for mo of conh33 why nh3 molecule is called a sigma donor ligand. Cobalt exists in the 3 oxidation state. Follow report by.
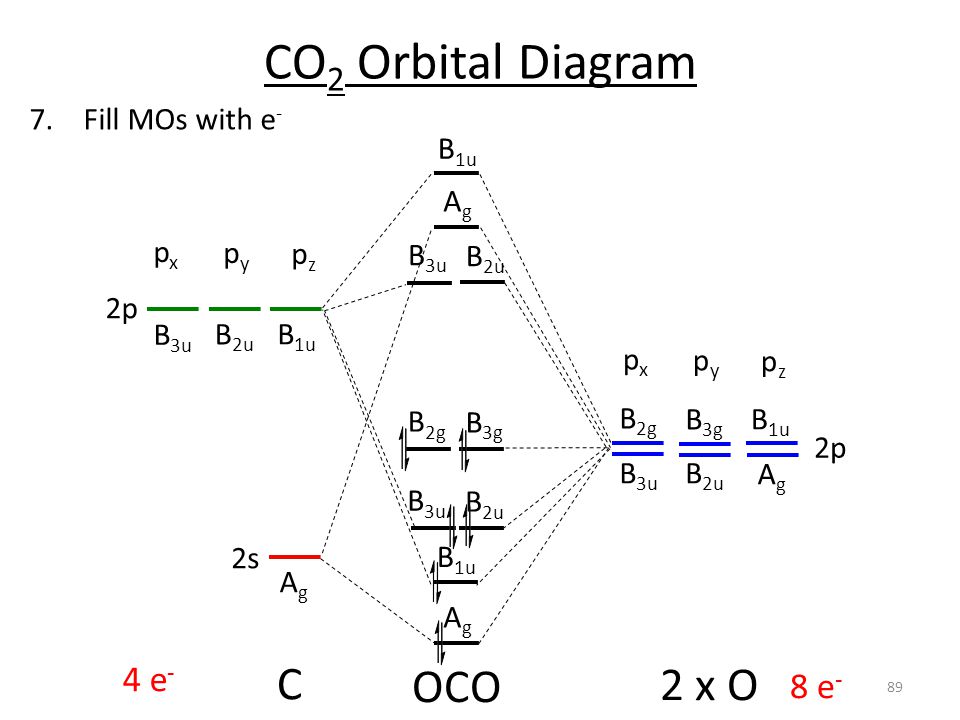
This is why there are 10 elements in each row of the d block. We now turn to a molecular orbital description of the bonding in ceo2. Fluorine ion is a weak ligand.
3 draw the molecular orbital diagram for mo of cof63. Such a theory is the so called ligand field theory lft which has its origin in the. The complex formation involves d orbitals of the outershell which give a high spin complex.
Log in join now secondary school. Smos for octahedral complexes 1. For a diatomic molecule the atomic orbitals of one atom are shown on the left and those of the other atom are shown on the right.
As a result the co3 ion will undergo sp3d2 hybridzation. 1 draw the molecular orbital diagram for mo theory of coco63. It cannot cause the pairing of the 3d electrons.
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram figure 9. In the both of water and methane the centre atom is a sp 3 state but the shape of the two molecules are different because in methane all electron are shared with hydrogen and thus forming tetrahedral shape and water h 2 o a water molecule has two pairs of bonded electrons and two unshared lone pairs. It so happens that the molecular orbital description of this molecule provided an explanation for a long standing puzzle that could not be explained using other bonding models.
Hence it shows bent shape or v shape. Molecular oxygen is paramagnetic. For octahedral complexes the crystal field splitting diagram will have its eg orbitals above the t2g orbitals and is.
Click here to get an answer to your question molecular orbital diagram of cof6 minus 3charge 1.
Remote Circuit Diagram, Molecular Orbital Diagram Of Cof 6 3 Complex With Six P Donor Ligands Download Table
- Crystal Field Theory Cft Detailed Explanation With Examples Videos
- Https Onlinelibrary Wiley Com Doi Pdf 10 1002 9780470687123
- Crystal Field Theory
Remote Circuit Diagram, 21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2
- Why Is Cof6 3 Paramagnetic Quora
- Chem 103 Lectures 7 8 Mo Diagrams And Magnetism Mo Diagram For A Simple Octahedral Complex Stacks Like This One Represent Degenerate Orbitals L L Course Hero
- Inorganic Chemistry Pages 651 700 Flip Pdf Download Fliphtml5
Remote Circuit Diagram, Chemistry The Central Science Chapter 24 Section 5
- Introduction To Inorganic Chemistry Coordination Chemistry And Crystal Field Theory Wikibooks Open Books For An Open World
- Crystal Field Theory Valence Bond Theory Cof6 3 Complex Ion Youtube
- 21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2
More From Remote Circuit Diagram
- Shelly 1 3 Way Wiring Diagram
- Floral Diagram Of Hamelia Patens
- Ats Circuit Diagram
- What Is The Lewis Structure For Ch4
- Labelled Diagram Of An Atom
Incoming Search Terms:
- Crystal Field Theory Cft Detailed Explanation With Examples Videos Labelled Diagram Of An Atom,
- Crystal Field Theory Calango Free Online Courses Labelled Diagram Of An Atom,
- Http Site Iugaza Edu Ps Skurdi Files 2015 02 Icb 21 Final Pdf Labelled Diagram Of An Atom,
- Molecular Orbital Theory Of Octahedral Complexes Labelled Diagram Of An Atom,
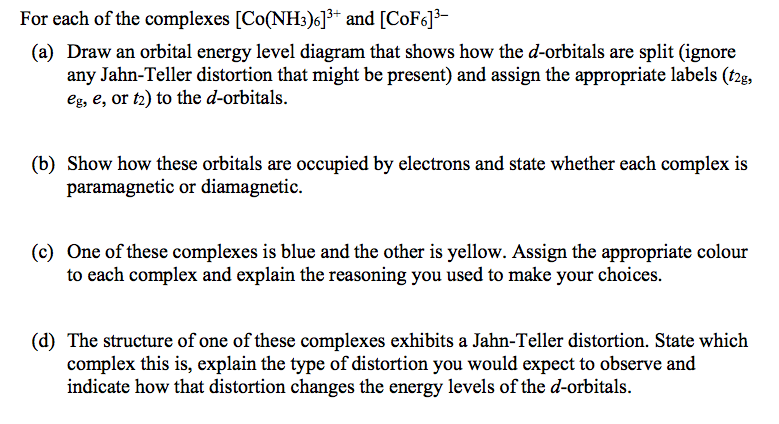
- Solved For Each Of The Complexes Co Nh3 6 3 And Cof6 3 Chegg Com Labelled Diagram Of An Atom,
- On The Basis Of Valence Bond Theory Explain The Oxidation State Hybridisation Geometry And Magnetic Nature Of Metal In Complex Cof6 3 Labelled Diagram Of An Atom,