Simple Electrolytic Cell Diagram, Simple Voltaic Cells Batteries Copper Zinc Cell Gcse Chemistry Ks4 Science Igcse O Level Revision Notes
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcquo3gm1br0f Ahpasbohw7sktmmbldv52bu6tmedf Derjxukp Usqp Cau
- Voltaic Cells Chemistry Libretexts
- Electrolytic Cells
- Electrochemical Cells Potentiostats
- Electrochemistry Article Khan Academy
- Definition Of Electrolytic Cell Chemistry Dictionary
- Electrolysis Chemistry For Majors
- Ib Chemistry Notes Voltaic Cells
- Electrolysis Concepts Chemistry Tutorial
- Electrochemical Cell Definition
Find, Read, And Discover Simple Electrolytic Cell Diagram, Such Us:
- Electrolysis And Simple Cell Teaching Resources
- Electrochemistry Electrochemical Cells
- Definition Of Voltaic Cell Chemistry Dictionary
- Chemistry Glossary Search Results For Irreversible Galvanic Cell
- Electrolytic Cell An Overview Sciencedirect Topics
If you are searching for Mccb Circuit Diagram you've come to the ideal location. We have 104 graphics about mccb circuit diagram adding pictures, photos, photographs, wallpapers, and more. In such webpage, we additionally have number of images available. Such as png, jpg, animated gifs, pic art, symbol, black and white, transparent, etc.
Simple electrolysis cell that students can label and annotate.

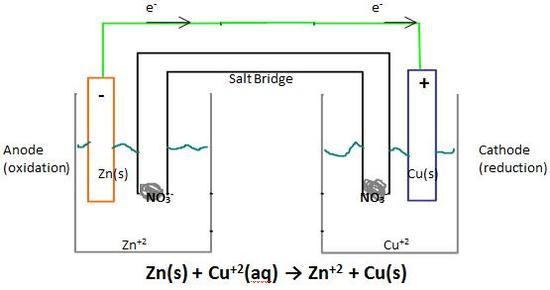
Mccb circuit diagram. An electrolytic cell uses electrical energy to drive a non spontaneous redox reaction. Electrolytic cells like galvanic cells are composed of two half cells one is a reduction half cell the other is an oxidation half cell. The electrodes are made of two different metals.
The process is known as electrolysis. The other category voltaic cells convert chemical potential energy to electrical energy. The purpose of this is usually to convert reactants into more useful products.
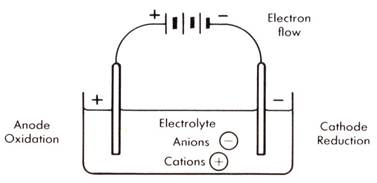
A simple electric cell consists of two electrodes two different metals and an electrolyte solution acid or alkali solution or salt solution how simple electric cells work. These cells are called electrolytic cells. Alternatively the cells which cause chemical reactions to occur in them when an electric current is passed through them are called electrolytic cells.
On your diagram show the direction in which current flows. Remember that conventional current flow is in the opposite direction to electron flow. A diagram detailing the different parts of an electrochemical cell is provided below.
Fill in the table below to summarise the information on galvanic and electrolytic cells. In our example the compound to be split is plain salt nacl. Diagram and working of an electrolytic cell molten sodium chloride nacl can be subjected to electrolysis with the help of an electrolytic cell as illustrated below.
It is first liquefied by heating it till the salt crystals melt. Electrolytic cells are one of two major categories of electrochemical cell. Simple electrolysis cell that students can label and annotate.
Simple electrolysis cell diagram no rating 0 customer reviews. Here two inert electrodes are dipped into molten sodium chloride which contains dissociated na cations and cl anions. Electrolytic cells use electrical work as source of energy to drive the reaction in the opposite direction.
It is possible to construct a cell that does work on a chemical system by driving an electric current through the system. This is the basic working principle of an electrolytic cell. This liquid is then pored in an electrolysis container.
Draw a simple diagram of the electrolytic cell. A simple cell is a device that converts chemical energy into electrical energy.
Mccb Circuit Diagram, What Is An Electrochemical Cell Structure Uses Video Lesson Transcript Study Com
- Electrolytic Cell An Overview Sciencedirect Topics
- Simple Cell Notation Construction Electrode Potential Chart Daniel Cell Gce A Level As A2 Chemistry Revision Notes Ks5
- Difference Between Galvanic Cells And Electrolytic Cells Difference Between
Mccb Circuit Diagram, Acids Practice Test 2
- Chemistry Glossary Search Results For Irreversible Galvanic Cell
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcquo3gm1br0f Ahpasbohw7sktmmbldv52bu6tmedf Derjxukp Usqp Cau
- Similarities And Differences Between Voltaic Cells And Electrolytic Cells Science Struck
Mccb Circuit Diagram, Labeled Diagram To Show The Electrolysis Of Acidified Water Forming Royalty Free Cliparts Vectors And Stock Illustration Image 47667616
- 20 3 Voltaic Cells Chemistry Libretexts
- Electrochemical Cell Components Cell Reactions Types Applications Significance
- Electrochemical Cell An Overview Sciencedirect Topics
More From Mccb Circuit Diagram
- Open Block Diagram Labview
- Sulfur Atom Diagram
- Negative Clamper Circuit Diagram
- Funny Venn Diagrams Covid
- Scientific Diagram Of A Plant
Incoming Search Terms:
- The Profile Of Current Density Between Electrodes In The Simple Download Scientific Diagram Scientific Diagram Of A Plant,
- Electrolytic Cells Video Lesson Transcript Study Com Scientific Diagram Of A Plant,
- Sch4u Electrochemistry Cell Reactions Scientific Diagram Of A Plant,
- Electrochemistry Electrolysis Electrolytic Cell Electroplating Youtube Scientific Diagram Of A Plant,
- Electrochemical Cells Potentiostats Scientific Diagram Of A Plant,
- Galvanic Cell Images Stock Photos Vectors Shutterstock Scientific Diagram Of A Plant,