Reaction Coordinate Diagram With Catalyst, The Graph Of The Effect Of Catalyst On Activation Energy Class 12 Chemistry Cbse
- Catalysis Fundamentals Chemical Engineering Page 1
- Effect Of Catalysts
- Energy Profile Chemistry Wikipedia
- What Is A Catalysts
- Fuck Biochemistry In 2 Ways Enzyme Mechanisms
- Lecture 9
- 12 7 Catalysis Chemistry
- Enzyme Catalysis 28 October 2014 Katja Dove Phd Candidate Department Of Biochemistry University Of Washington Please Ppt Download
- Undergraduate Chemistry Students Misconceptions About Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing
- 12 7 Catalysis Chemistry 112 Chapters 12 17 Of Openstax General Chemistry
Find, Read, And Discover Reaction Coordinate Diagram With Catalyst, Such Us:
- Reaction Coordinate Diagram For An Enzyme Catalyzed Reaction Download Scientific Diagram
- Solved Using The Reaction Coordinate Diagram Below Which Chegg Com
- Https Go Nature Com 2yu0ir1
- Lecture 9
- Reaction Energy Rates Worksheet
If you re looking for Single Phase Submersible Starter Connection you've arrived at the ideal place. We ve got 104 images about single phase submersible starter connection adding pictures, pictures, photos, backgrounds, and more. In such web page, we also provide number of graphics available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.
Http Www Mrfischer Com Wp Content Uploads Reaction Energy And Reaction Rates0001 Pdf Single Phase Submersible Starter Connection

Reaction Coordinate Diagram For An Enzyme Catalyzed Reaction Download Scientific Diagram Single Phase Submersible Starter Connection
The two reaction diagrams here represent the same reaction.
Single phase submersible starter connection. In chemistry a reaction coordinate 1 is an abstract one dimensional coordinate which represents progress along a reaction pathway. The diagram below is called a reaction coordinate diagram. A according to the lock and key model the shape of an enzymes active site is a perfect fit for the substrate.
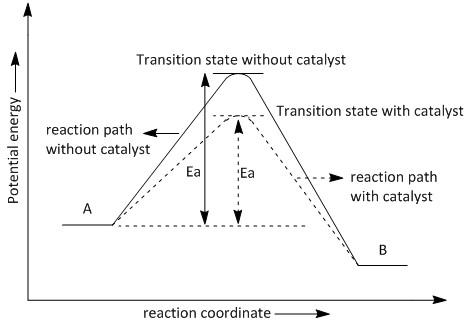
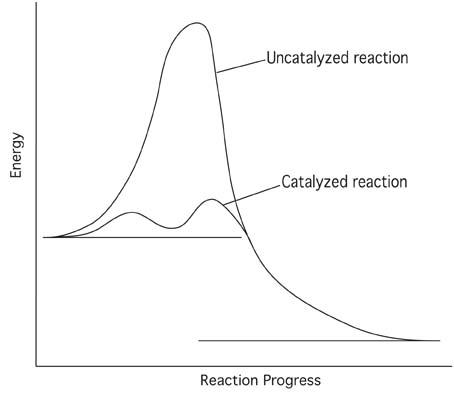
Diagram of a catalytic reaction showing the energy niveau depending on the reaction coordinate. In an energy diagram the vertical axis represents the overall energy of the reactants while the horizontal axis is the reaction coordinate tracing from left to right the progress of the reaction from starting compounds to final products. Ron rusay a reaction coordinate energy diagram thermodynamic quantities.
The fully filled in reaction coordinate diagram is displayed below. Struggling to understand catalysts or reaction coordinate diagrams or maybe trying to determine a reactions rate law from its mechanism. A catalyst can have a profound inuence lowering the energy of activation and altering the mechanism.
One without a catalyst and one with a catalyst. Sketch a single reaction coordinate diagram for the reaction pathways catalyzed by catalysts a and b with clear labels provided for the reactant state energy and the two transition state energies. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams.
It shows how the energy of the system changes during a chemical reaction. The arrow marked in the question represents the activation energy which is the energy barrier that must be overcome in order for the reactants to form products. This potential energy diagram shows the effect of a catalyst on the activation energy.
This is an exothermic reactionheat is given off and should be favorable from an energy standpoint. Geometry and steric effects impact the reaction rate. Identify which diagram suggests the presence of a catalyst and determine the activation energy for the catalyzed reaction.
Energy diagram for a two step reaction mechanism complete energy diagram for two step reaction a two step reaction mechanism the transition states are located at energy maxima. The presence of nitric oxide no influences the rate of decomposition of ozone. This ensures that the metals in the catalyst are fully active even before the automobile exhaust is hot enough.
Each step has its own delta h and answer the activation energy would be lower. Solution a catalyst does not affect the energy of reactant or product so those aspects of the diagrams can be ignored. For a catalysed reaction the activation energy is lower.
Your answer should mention boltzmann energy distributions. The new diagram now looks like the one shown below. D provide a physical explanation why the rate constant has an exponential dependence on temperature hint.
The reactive intermediate b is located at an energy minimum.
Single Phase Submersible Starter Connection, 12 7 Catalysis Chemistry
- Http Thelaralab Weebly Com Uploads 2 2 5 4 22540374 Reaction Pathways Worksheet Answers Pdf
- Reaction Coordinate Diagrams
- The Graph Of The Effect Of Catalyst On Activation Energy Is Given
Single Phase Submersible Starter Connection, Reaction Coordinate Diagram For Wilkinson S Catalyst Interaction Download Scientific Diagram
- Reaction Coordinate
- Reaction Coordinate Diagram Showing The Working Principle Of A Catalyst Download Scientific Diagram
- Oneclass Physical Origins Of Enzyme Catalysis 2 Draw A Reaction Coordinate Diagram For A One Step R
Single Phase Submersible Starter Connection, Http Www Mrfischer Com Wp Content Uploads Reaction Energy And Reaction Rates0001 Pdf
- Thermodynamics And Kinetics
- Labeling Parts Of A Reaction Coordinate Diagram Youtube
- Chem 245 Enzyme Kinetics
More From Single Phase Submersible Starter Connection
- Diagram Of Electron Configuration
- 2002 Ford Taurus Fuse Box Diagram
- Onion Cell Diagram Class 9
- Energy Flow Diagram Physics
- Labeled Electrolytic Cell Diagram
Incoming Search Terms:
- 12 7 Catalysis Chemistry 112 Chapters 12 17 Of Openstax General Chemistry Labeled Electrolytic Cell Diagram,
- The Graph Of The Effect Of Catalyst On Activation Energy Is Given Labeled Electrolytic Cell Diagram,
- Activation Energy And Catalysts Ppt Download Labeled Electrolytic Cell Diagram,
- 1 Schematic Reactivity Curves Along A Reaction Coordinate For An Download Scientific Diagram Labeled Electrolytic Cell Diagram,
- Description Of The Reaction Coordinate Youtube Labeled Electrolytic Cell Diagram,
- Explain With The Help Of Potential Energy Barrier How Br Does A Labeled Electrolytic Cell Diagram,