Reaction Coordinate Diagram Kinetic Vs Thermodynamic, 14 3 Kinetic Vs Thermodynamic Control Of Reactions Chemistry Libretexts
- Using A Free Energy Diagram To Explain Thermodynamic Vs Kinetic Products Youtube
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcq9bwwrhzaup2bbdgfnh Vmq2qdbtvoxngdytbklmxiosfza88f Usqp Cau
- Chem 440 Enzyme Kinetics
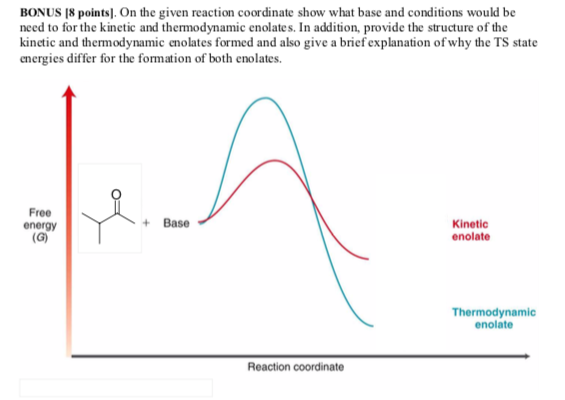
- Solved Bonus 8 Points On The Given Reaction Coordinate Chegg Com
- Thermodynamically And Kinetically Controlled Reactions In Biocatalysis From Concepts To Perspectives Marsden 2019 Chembiochem Wiley Online Library
- 6 6 Reaction Coordinate Diagrams Chemistry Libretexts
- Energy Profile Chemistry Wikiwand
- Gibbs Energy As A Function Of The Reaction Coordinate Of A Under Download Scientific Diagram
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcq9bwwrhzaup2bbdgfnh Vmq2qdbtvoxngdytbklmxiosfza88f Usqp Cau
- The Collision Model Of Chemical Kinetics
Find, Read, And Discover Reaction Coordinate Diagram Kinetic Vs Thermodynamic, Such Us:
- Thermodynamically And Kinetically Controlled Reactions In Biocatalysis From Concepts To Perspectives Marsden 2020 Chemcatchem Wiley Online Library
- 5 3 Reaction Coordinate Diagrams Organic Chemistry 1 An Open Textbook
- Solved 8 6 Pts Reactants A And B Can Undergo 2 Differe Chegg Com
- The Collision Model Of Chemical Kinetics
- Thermodynamic Versus Kinetic Reaction Control Wikipedia
If you are looking for Au Orbital Diagram you've reached the perfect place. We have 104 graphics about au orbital diagram adding images, photos, photographs, wallpapers, and much more. In these webpage, we additionally have variety of graphics out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.
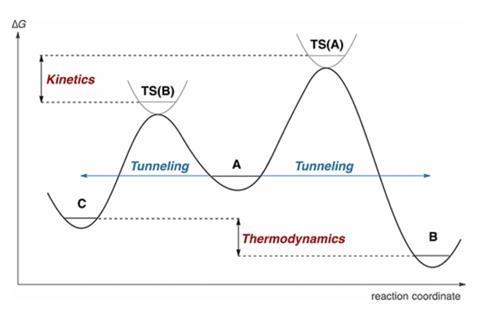
If the transition states leading to the formation of cec eg t c1 and t c2 were to be higher in energy than that leading to ceb eg t b1 and t b2 then.

Au orbital diagram. Note that the x axis is officially titled reaction coordinate this is a somewhat complicated measure of how far the reaction has progressed or how much of the reaction has happened. Understanding the reaction coordinate diagram. Kinetic factorsthe following simple reaction coordinate diagram provides a basis for the key issues about kinetic and thermodynamic control.
Kinetic and thermodynamic control. It is often more convenient to assume the reaction is going at a somewhat constant rate and think of the x axis simply as time. At a low temperature the amount of energy in the reaction is not.
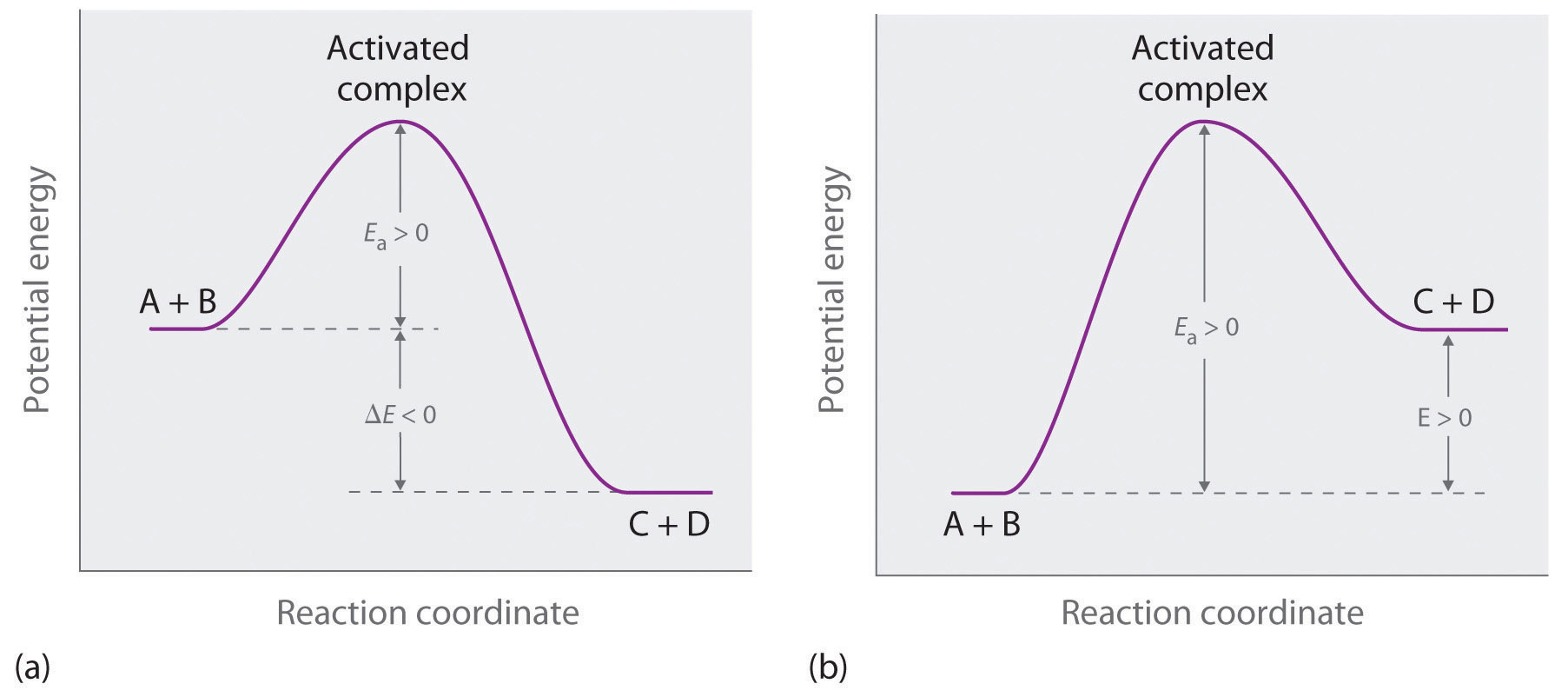
Reaction coordinate diagram of ozone photolysis the reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. Thermodynamic factorsthe rate of product formation ie.
A measure of freedom of motion dgo dho tdso dgdhds de are state. The relative stability of the products ie. Together the products o 2 and atomic o have a higher energy than the reactant o 3 and energy must be added to the system for this reaction.
Qualitatively the reaction coordinate diagrams one dimensional energy surfaces have numerous applications. This means that there is not enough energy to overcome the activation energy of the thermodynamic product even though it is the more stable product. The heat given off or absorbed during a reaction entropy dso.
In the gure below the activation energy ea is that critical minimum energy in a chemical reaction required by reactants to be. Major product at low temperatures 1 2 bromobutene. Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivitythe distinction is relevant when product a forms faster than product b because the activation energy for product a is lower than.
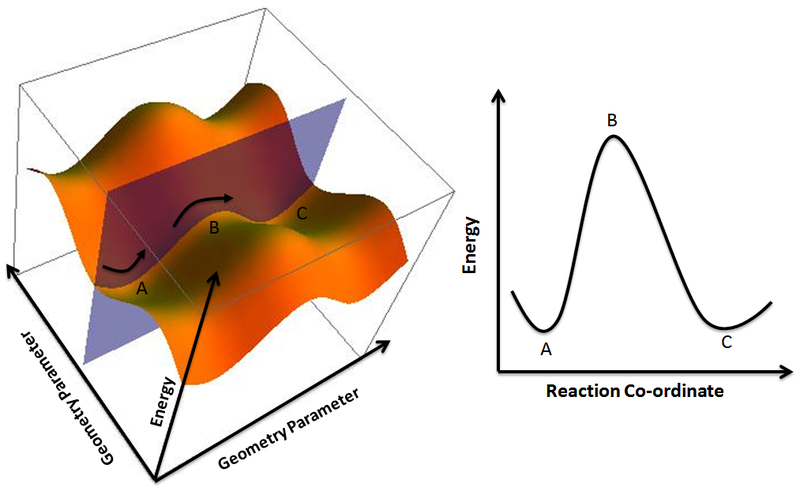
The main product at low temperatures is kinetically controlled. In an energy diagram the vertical axis represents the overall energy of the reactants while the horizontal axis is the reaction coordinate tracing from left to right the progress of the reaction from starting compounds to final products. Chemists use reaction coordinate diagrams as both an analytical and pedagogical aid for rationalizing and illustrating kinetic and thermodynamic events.
Thermodynamics vs kinetics overview a general reaction coordinate diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the gure below. Ron rusay a reaction coordinate energy diagram thermodynamic quantities gibbs standard free energy change dgo enthalphy dho.
Au Orbital Diagram, Reaction Coordinate Diagrams College Chemistry
- Thermodynamics And Kinetics Driving Quality In Drug Discovery European Pharmaceutical Review
- Http Epgp Inflibnet Ac In Epgpdata Uploads Epgp Content Chemistry 05 Organic Chemistry Ii 03 Thermodynamic And Kinetic Requirements Of A Reaction Et 5547 Et Et Pdf
- Reaction Coordinate Diagrams
Au Orbital Diagram, Energy Profile Chemistry Wikipedia
- Gibbs Free Energy In Reaction Profiles Chemistry Community
- Solved Bonus 18 Points On The Given Reaction Coordinate Chegg Com
- The Network Simulation Method A Useful Tool For Locating The Kinetic Thermodynamic Switching Point In Complex Kinetic Schemes Physical Chemistry Chemical Physics Rsc Publishing
Au Orbital Diagram, Transition State Theory Wikipedia
- Kinetic And Thermodynamic Control In The Diels Alder Reaction
- 6 Reaction Coordinate Diagram Viziscience Interactive Labs
- Catalysis Chemistry For Majors
More From Au Orbital Diagram
- 2003 Nissan Sentra Fuse Box Diagram
- Venn Diagram Respiration And Photosynthesis
- Shark Circulatory System Diagram
- Algorithm Flowchart Code
- Eulerape
Incoming Search Terms:
- Addition Of Hydrogen Bromide To 1 3 Butadiene Thermodynamic And Kinetic Control Chemistry Stack Exchange Eulerape,
- Reaction Coordinate Diagrams Eulerape,
- Transition State Theory Wikipedia Eulerape,
- Http Epgp Inflibnet Ac In Epgpdata Uploads Epgp Content Chemistry 05 Organic Chemistry Ii 03 Thermodynamic And Kinetic Requirements Of A Reaction Et 5547 Et Et Pdf Eulerape,
- Reaction Coordinate Wikipedia Eulerape,
- Electrophilic Attack On Conjugated Dienes Kinetic And Thermodynamic Control Chemistry Libretexts Eulerape,