Molecular Orbital Theory F2, Write The Molecular Electronic Configuration Of F2 And C2 Draw The Energy Level Diagram Calculate Brainly In
- Molecular Orbital Theory Lecture
- Use The Molecular Orbital Energy Level Diagram To Show That N 2
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcqny 3 Pa3 Xvpunc1evhr2fd9v2vr7xzxotxl4ppzcmbaqa3hx Usqp Cau
- Mo Diagram 2 F2 Youtube
- Molecular Orbital Diagram Wikipedia
- Molecular Orbital Diagrams O2 F2 Youtube
- Multiple Bonds Bond Energies Lengths Molecular Orbital Theory General Chemistry Lecture 1140 Dr Sundin Uw Platteville
- Http Docshare04 Docshare Tips Files 24504 245047568 Pdf
- Pin On Chemistry
- Https Portal Miracosta Edu Students Chemistry Resource Center Tests 110 Exam5 Mccorkle Sp16 Key Pdf
Find, Read, And Discover Molecular Orbital Theory F2, Such Us:
- Molecular Orbital Diagram Part 2 Chemical Bonding And Molecular Structure Unacademy
- Ie Organic Lecture 3 The Mo Diagram Of F2 Youtube
- Molecular Orbitals For Difluorine
- Following Diatomic Omonuclear Molecules H2 B2 C2 N2 O2 Ne2
- Beyond The Molecular Orbital Conception Of Electronically Excited States Through The Quantum Theory Of Atoms In Molecules Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C4cp00431k
If you are looking for What Is Flowchart In Programming you've come to the perfect place. We ve got 104 images about what is flowchart in programming adding images, pictures, photos, wallpapers, and much more. In these page, we additionally provide variety of graphics out there. Such as png, jpg, animated gifs, pic art, logo, black and white, transparent, etc.
Https Switkes Chemistry Ucsc Edu Teaching Chem1b Handouts Diatomicmoleculeorbitalenergylevels Pdf What Is Flowchart In Programming
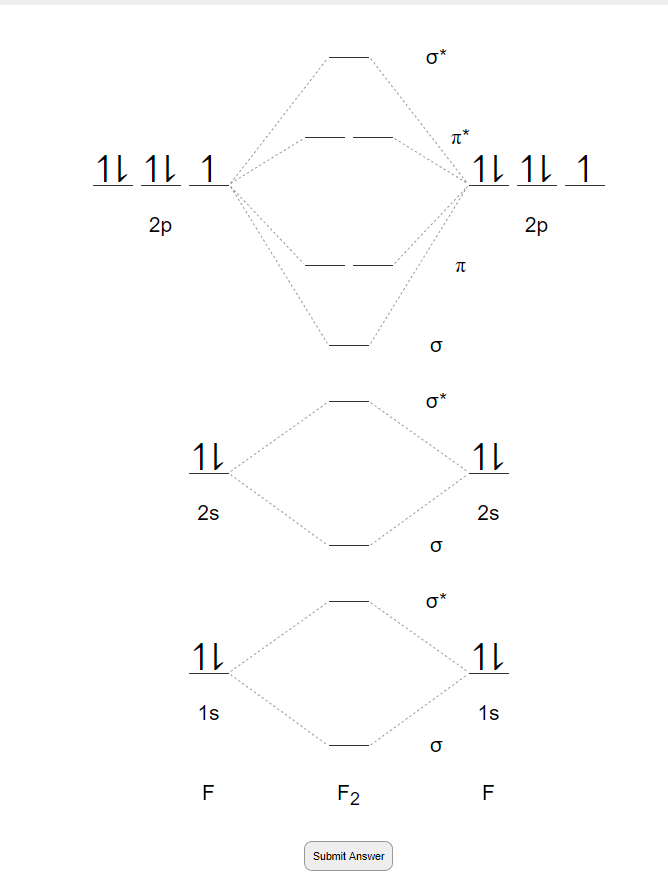
Molecular orbital theory mo theory provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule.
What is flowchart in programming. Notice how the sg2p or the s2pz molecular orbital dips down below the p molecular orbital energies after n2. The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. In more advanced theory every single atomic orbital can be considered to some extent in every molecular orbital.
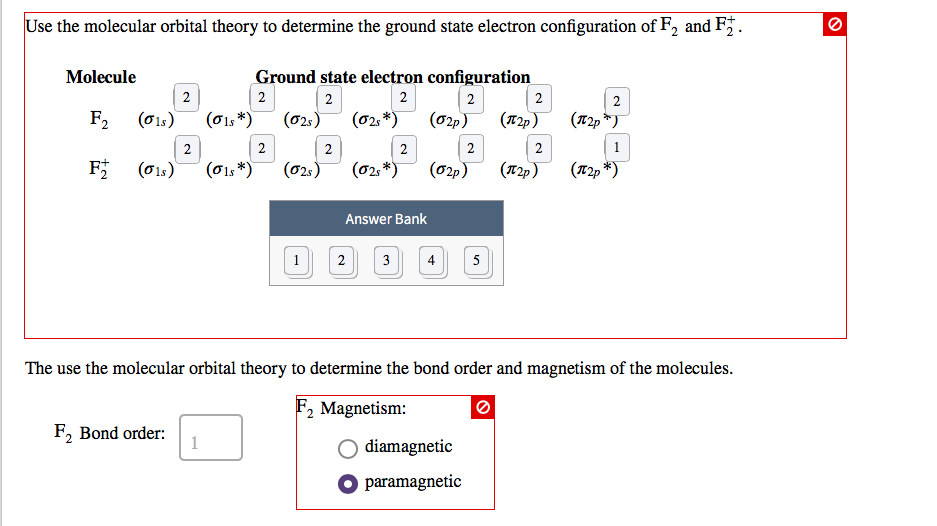
This is because. Also calculate the bond order and predict the magnetic behaviour. A the bond order in f2 can be shown to be equal to 1.
The relative energies of the sigma orbitals drop below that of the pi orbitals. B there are more electrons in the bonding orbitals than in the antibonding orbitals. It also explains the bonding in a number of other molecules such as violations of the octet rule and more molecules with more complicated bonding beyond the scope of this text that are difficult to describe with lewis structures.
If you use the aufbau process to populate the sigma pi pi and sigma orbitals of these species you will find that f2 has bond order 15 f2 has bond order 10 and f2 has bond order 05. For the ion f2a draw the molecular orbital diagramb calculate the bond orderc would this ion existd write the electron configuration of the ion. Since the antibonding molecular orbital forces the electron to spend most of its time away from the area between the nuclei placing an electron in this orbital makes the molecule less stable.
Placing an electron in this orbital therefore stabilizes the h 2 molecule. C all electrons in the mo electron configuration of f2 are paired. Since f2 is after n2 in the second row of the periodic table where these effects are not present the orbital energy ordering is normal.
With the help of molecular orbital theory draw the molecular orbital energy level diagram for molecule. In general the molecular orbital energies follow these rules. However the molecular orbitals are greatly simplified if we only consider localized atomic orbitals around the two bonded atoms ignoring the others our approach above.
In o 2 and f 2 there is a crossover of the sigma and the pi ortbials. The number of antibonding electron pairs in.
What Is Flowchart In Programming, Delocalized Bonding And Molecular Orbitals
- Diatomic Species Mo Theory Chemogenesis
- Molecular Orbitals
- Diatomic Species Mo Theory Chemogenesis
What Is Flowchart In Programming, Draw The Molecular Orbital Diagram For F2 And Find Out The Bond Order Brainly In
- Mo Theory
- Antibonding Molecular Orbitals Yahoo Dating
- Https Portal Miracosta Edu Students Chemistry Resource Center Tests 110 Exam5 Mccorkle Sp16 Key Pdf
What Is Flowchart In Programming, Molecular Orbital Diagram F2 Untpikapps Free Photos
- Https Authors Library Caltech Edu 105209 10 Tr000574 03 Chapter 3 Pdf
- Pin On Chemistry
- Answered What Is The Bond Order Of F2 Bartleby
More From What Is Flowchart In Programming
- 2006 Ford F150 Fuse Diagram
- Free Body Diagram Definition Class 11
- What Is The Correct Lewis Structure For Hydrogen Chloride Hcl
- Diagram Of Angiosperm Ovule
- Understanding Block Diagrams
Incoming Search Terms:
- Most Important Resonant Bonding Patterns For The C2 N2 O2 And F2 Download Scientific Diagram Understanding Block Diagrams,
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcqonohwn4axymthmkltmta9lvztg8iw5bsphdh0ruiggkmtwfim Usqp Cau Understanding Block Diagrams,
- Ppt Molecular Orbital Theory Powerpoint Presentation Free Download Id 5569271 Understanding Block Diagrams,
- Chemistry Xl 14a Molecular Shape And Structure Ppt Video Online Download Understanding Block Diagrams,
- Why There Is A Change In Sequence Of Energies For O2 F2 Ne2 In Molecular Orbital Theory Chemistry Chemical Bonding 11761815 Meritnation Com Understanding Block Diagrams,
- Write The Molecular Electronic Configuration Of F2 And C2 Draw The Energy Level Diagram Calculate Brainly In Understanding Block Diagrams,