Galvanic Vs Electrolytic Cell Diagram, Electrochemical Cell Parts
- Electrolysis And Electrolytic Cell Test Quiz Proprofs Quiz
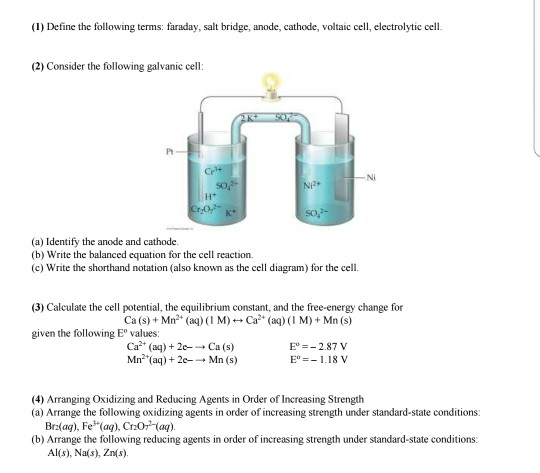
- How Electrochemical Cells Work Electrochemical Cell Galvanic Cell Electrochemistry
- Electrochemistry Mcat Review
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcq5iltxokxju Ftgnmxbx4iwgyi1x4rt2yygau8sy5raxigpwtt Usqp Cau
- Electrochemistry Mcat Review
- Galvanic Cell Biochemreview
- What Is The Difference Between Electrolytic And Galvanic Cell Quora
- Electrochemistry Electrolysis Ppt Video Online Download
- Galvanic Versus Electrolytic Cells Youtube
- 10 Key Difference Between Electrolytic Cell And Electrochemical Cell
Find, Read, And Discover Galvanic Vs Electrolytic Cell Diagram, Such Us:
- Galvanic Or Voltaic Cell Definition
- Electrochemistry Article Khan Academy
- Electrochemistry Mcat Review
- Difference Between Galvanic And Electrolytic Cell Definition How They Work
- Electrochemical Or Galvanic Or Voltaic Cells Tutors 4 You
If you re searching for Audi A7 Fuse Box Diagram you've arrived at the ideal place. We ve got 104 graphics about audi a7 fuse box diagram adding pictures, photos, pictures, backgrounds, and more. In such page, we additionally provide variety of images available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.
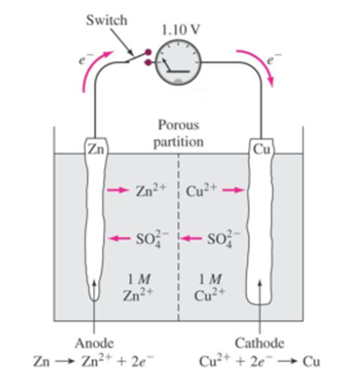
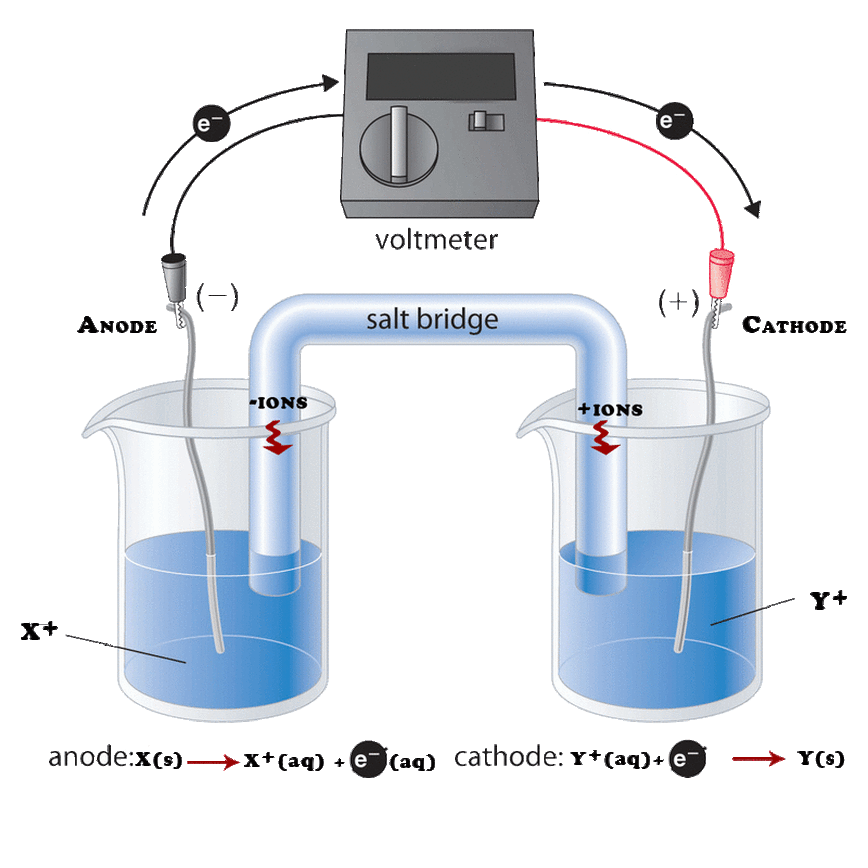
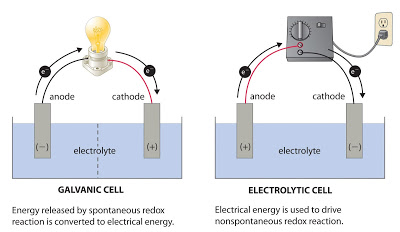
In an electrolytic cell the direction of current is opposite of that in the galvanic cells.
Audi a7 fuse box diagram. Summary of galvanic vs. Galvanic cell vs electrolytic cell. Electrolytic cell an electrochemical cell is composed of two half cells or electrodes whose contact is made via an electrolyte ionic conductor.
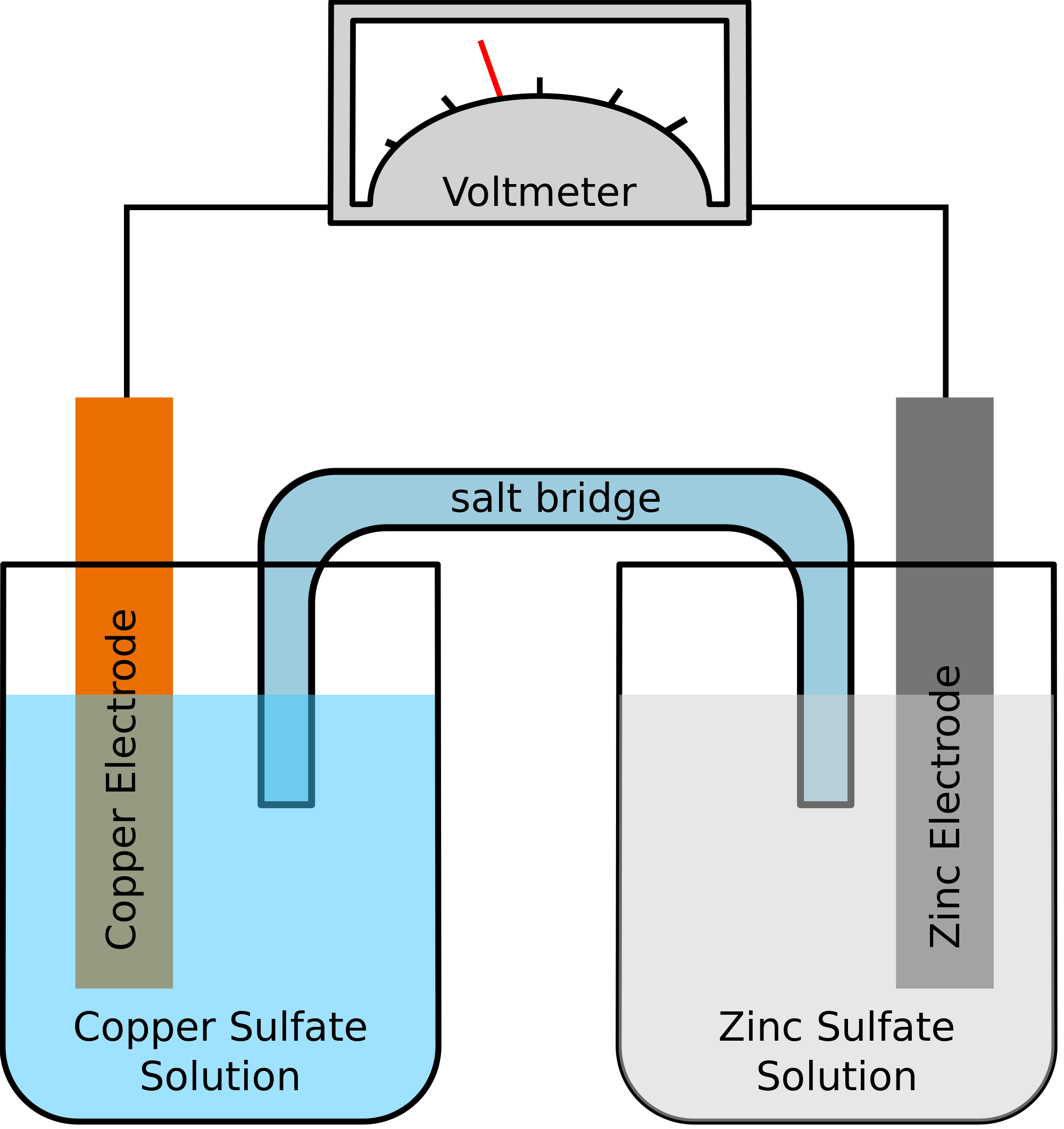
Use pdf export for high quality prints and svg export for large sharp images or embed your diagrams anywhere with the creately viewer. Half cells if separated can be joined by a salt bridge concentrated solution of electrolytes in agar agar gel. An electrolytic cell is a cell that uses an electric current for the progression of a chemical reaction.
Creately diagrams can be exported and added to word ppt powerpoint excel visio or any other document. Lastly once dead galvanic cells cannot be revived or recharged. It explains how to identify the anode and th.
Difference between galvanic and electrolytic cell definition. A galvanic cell converts chemical energy into electrical energy. An electrolytic cell converts electrical energy into chemical energy.
Copy of voltaic vs electrolytic cells you can edit this template and create your own diagram. This chemistry video tutorial explains how to draw galvanic cells and voltaic cells given the overall reaction. Here the redox reaction is spontaneous and is responsible for the production of electrical energy.
Descriptions a voltaic or galvanic cell. A voltaic cell is an electrochemical device that produces electrical energy from a spontaneous reduction oxidation reaction also called a redox reactiona redox reaction is a chemical reaction wherein one reactant loses electrons oxidation reaction and the other reactant gains electrons reduction reaction. Electrolytic cells and galvanic cells both involve the transfer of electrons and energy.
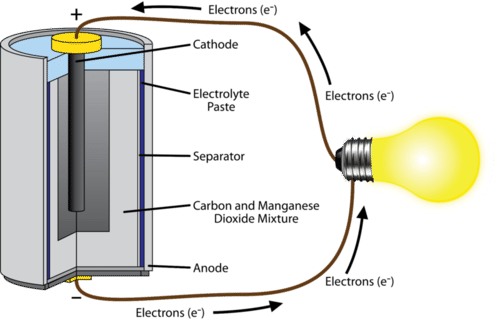
Electrochemical cell galvanic cell electrolytic cell. This is why one must change the batteries in an alarm clock or remote control from time to time. A galvanic cell is an electrochemical cell that can produce electricity with the help of a chemical reaction.
In this lesson we will go through the similarities and differences between the two.
Audi A7 Fuse Box Diagram, What Is The Difference Between Electrolytic And Galvanic Cell Quora
- Galvanic And Electrolytic Cells
- How Galvanic Or Voltaic Cells Work
- Electrochemical Cell Parts
Audi A7 Fuse Box Diagram, Galvanic Or Voltaic Cell Definition
- Difference Between Galvanic And Electrolytic Cell Definition How They Work
- Electrochemical Cells
- Electrolytic Cell And Electrochemical Cell Their Characteristics
Audi A7 Fuse Box Diagram, Describing Electrochemical Cells
- Galvanic Cell Wikipedia
- Galvanic Cell Diagrams Chemistry Tutorial
- Galvanic And Electrolytic Cells Electrochemical Reactions Siyavula
More From Audi A7 Fuse Box Diagram
- Anb Venn Diagram
- Stomata And Guard Cells Diagram
- Half Wave Rectifier Diagram
- Semi Trailer Pigtail Wiring Diagram
- The Two Brothers Plot Diagram
Incoming Search Terms:
- Voltaic Cells The Two Brothers Plot Diagram,
- Aluminium Graphite Anode Electrochemical Cell Used For Carbon Dioxide Download Scientific Diagram The Two Brothers Plot Diagram,
- Describing Electrochemical Cells The Two Brothers Plot Diagram,
- Electrochemical Cells The Two Brothers Plot Diagram,
- Sch4u Electrochemistry Cell Reactions The Two Brothers Plot Diagram,
- Voltaic Vs Electrolytic Cells Editable Venn Diagram Template On Creately The Two Brothers Plot Diagram,