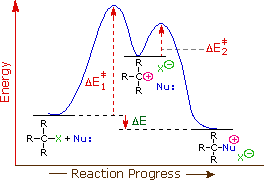
Energy Profile Diagram Of E1 And E2 Reaction, 7 4 Effects Of Solvent Leaving Group And Nucleophile On Unimolecular Substitution Chemistry Libretexts
- Solved Organic Chemistry Review Questions These Are Not Chegg Com
- A The Astrophysical S Factor Of The E2 Transition And B The S E2 S Download Scientific Diagram
- E2 Reaction
- E1 And E2 Reactions Organic Chemistry Socratic
- Reaction Energy Diagram Sn2 Youtube
- Sn1 Mechanism An Overview Sciencedirect Topics
- Solved Question 40 1 Point E1 E2 T E3 The Figure S Chegg Com
- Sn1 Reaction Energy Diagram Youtube
- A Generalised Energy Profile Diagram For Kinetic Versus Thermodynamic Product Reaction Organic Chemistry Chemistry Physical Chemistry
- 7 18 Comparison Of E1 And E2 Reactions Chemistry Libretexts
Find, Read, And Discover Energy Profile Diagram Of E1 And E2 Reaction, Such Us:
- 7 18 Comparison Of E1 And E2 Reactions Chemistry Libretexts
- Sn1 Reaction Energy Diagram Youtube
- Solved What Reaction Profile S Would Follow The Represen Chegg Com
- Elimination Reaction Wikipedia
- E1 Mechanism Kinetics And Substrate Video Khan Academy
If you are searching for 2 Way Switch Wiring Diagram Pdf you've come to the ideal place. We have 104 graphics about 2 way switch wiring diagram pdf including images, photos, pictures, backgrounds, and much more. In these web page, we also have number of images out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.
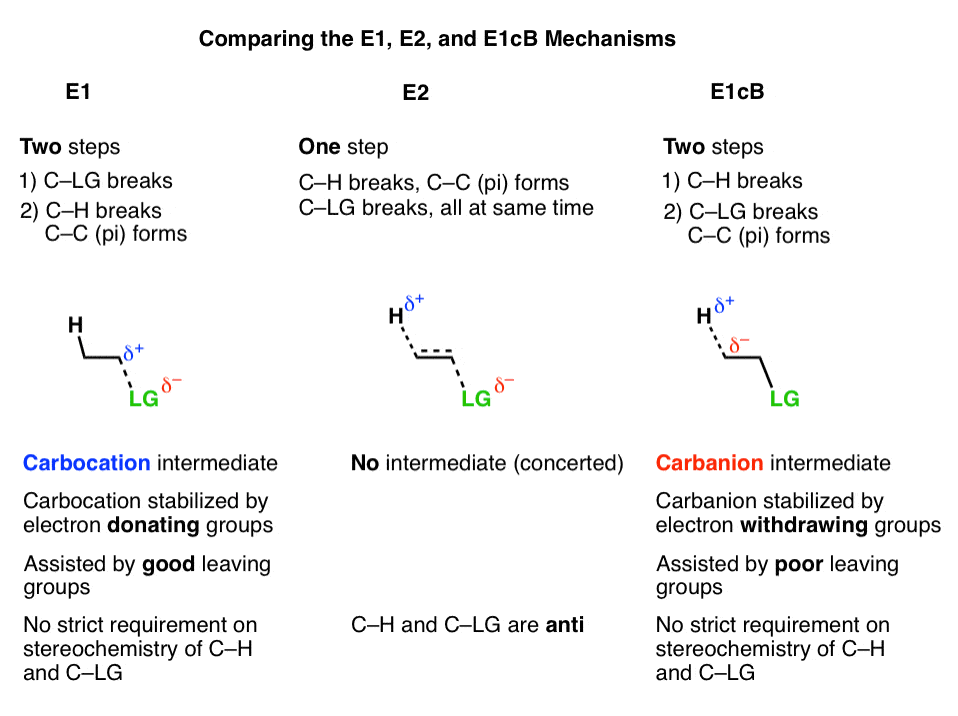
E2 reactions are concerted and occur faster whereas e1 reactions are step wise and occur slower and at a higher energy cost generally.
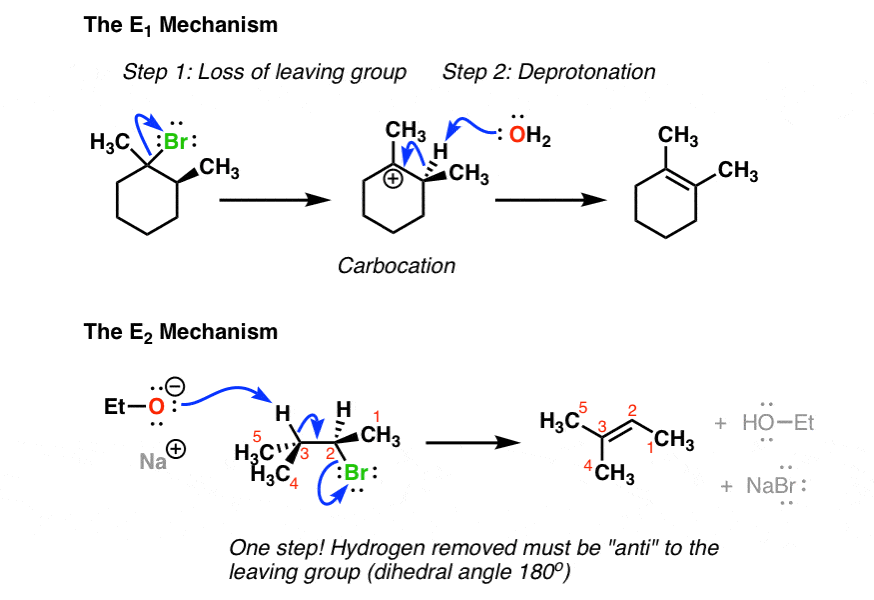
2 way switch wiring diagram pdf. The e1 and s n 1 reactions always compete and a mixture of substitution and elimination products is obtained. Below is a mechanistic diagram of an elimination reaction by the e2 pathway. Due to e1s mechanistic behavior carbocation rearrangements can occur in the intermediate such that the positive charge is relocated on the most stable carbon.
Stereochemistry of e1 and e2 reactions for an e1 reaction an intermediate carbocation is formed and so the stereochemistry of the starting material will not determine the stereochemistry of the final product. The slow step is unimolecularinvolving only the alkyl halide. The products formed though will favor having the two bulky group on the opposites side of the double bond the e alkene.
More bonds are being broken and formed with the possibility of a continuum of states in which the extent of ch and cx bond breaking and cc bond making varies. Draw an energy diagram for an e1 reaction. If a reaction occurs draw the products and write whether the mechanism that occurs is e2 sn2 or sn1e1.
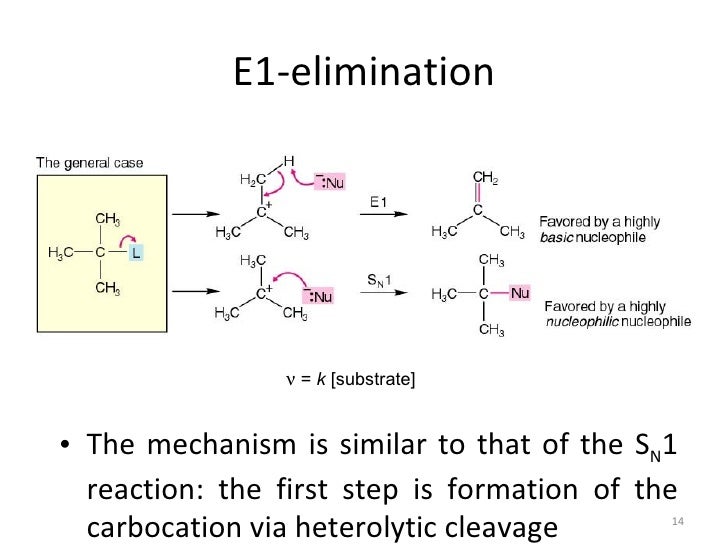
The energy diagram for sn2 and e2 will just be a one simple curve because there is no intermediate. In s1 and e1 there is a formation of a carbocation intermediate. E1 indicates a elimination unimolecular reactionthe e1 reaction proceeds via a two step mechanismthe bond to the leaving group breaks first before thep bond is formed.
Lets break down the steps of the e1 reaction and characterize them on the energy diagram. H3c ch c h2. The other two elimination reactions are e1 and e2 reactions.
Alkene alkynes or similar heteroatom variations such as carbonyl and cyano will form. E2 reactions of cyclic compoundsthe e2 reaction of menthyl chlorideviolates zaitsevs rule 41. An energy diagram for the single step bimolecular e2 mechanism is shown below.
All elimination reactions involve the removal of two substituents from a pair of atoms in a compound. The dehydrohalogenation of ch33ci with h2o to formch32cch2 can be used to illustrate the e1 mechanism. Loss of he leaving group.
We should be aware that the e2 transition state is less well defined than is that of s n 2 reactions. Both sn2 and e2. If no reaction occurs write no reaction.
E1 reactions of cyclic compoundswhen a cyclohexyl chloride undergoes an e1reaction there is no requirement that thetwo groups to be eliminated be diaxial 42. E1 a two step mechanism. 30 points i o br ch3oh br o br o o i o i ch3oh br br br ch3oh i o ch3ch2ch2oh dmf.
Sn2 and e2 is a concerted mechanism in which a bond is broken and another one forms at the same time.
2 Way Switch Wiring Diagram Pdf, Alkyl Halide
- Elimination Rxn Predict The Reaction Pathway Main Products For E2 And E1 Draw Reaction Mechanism For E1 Design Synthetic Pathway Based On Mechanism Ppt Download
- Elimination Reaction Wikipedia
- Gibbs Free Energy Diagram For The Propene Formation Over H Zsm 5 Via E1 Download Scientific Diagram
2 Way Switch Wiring Diagram Pdf, Alkyl Halides Nucleophilic Substitution And Elimination Ppt Video Online Download
- E1 Reaction Mechanism And E1 Practice Problems
- Media Portfolio
- Sn1 Mechanism An Overview Sciencedirect Topics
2 Way Switch Wiring Diagram Pdf, Elimination Reaction Wikipedia
- Sn1 Reaction Energy Diagram Youtube
- E1 Reaction Mechanism And E1 Practice Problems
- Elimination Reaction Chemical Reaction Britannica
More From 2 Way Switch Wiring Diagram Pdf
- 2011 Toyota Camry Fuse Box Diagram
- Three Way Switch Diagram With Dimmer
- Marchantia Sporophyte Diagram
- Harvard Architecture Block Diagram
- Heart Valve Names Diagram
Incoming Search Terms:
- Comparing E2 E1 Sn2 Sn1 Reactions Video Khan Academy Heart Valve Names Diagram,
- Sn2 Reaction Organic Chemistry Video Clutch Prep Heart Valve Names Diagram,
- Sn1 Reaction Energy Diagram Youtube Heart Valve Names Diagram,
- 7 18 Comparison Of E1 And E2 Reactions Chemistry Libretexts Heart Valve Names Diagram,
- E2 Reactions Video Elimination Reactions Khan Academy Heart Valve Names Diagram,
- Reaction Energy Diagram Sn2 Youtube Heart Valve Names Diagram,