Energy Profile Diagram For Exothermic And Endothermic Reaction, Chemical Reactions And Energy Ck 12 Foundation
- Energy Level Diagram For Exothermic Reactions Ppt Download
- Igcse Chemistry 4 14 Represent Exothermic And Endothermic Reactions On A Simple Energy Level Diagram
- The Cold Pack A Chilly Example Of An Endothermic Reaction Let S Talk Science
- Chemistry M4 Energetics
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcran7ndusfniu97xqcjknjgdrdzsn09uuclo20act4xin18ezr0 Usqp Cau
- C2 5 Exothermic And Endothermic Reactions Secondary Science 4 All
- How To Draw Label Enthalpy Diagrams Video Lesson Transcript Study Com
- Reaction Energy Profiles Activation Energy Exothermic Endothermic Reactions Catalysed Uncatalysed Reactions Gcse Notes Ks4 Science Igcse O Level Chemistry Revision
- Energy Profile Diagram Lectures Notes Site
- 18 4 Potential Energy Diagrams Chemistry Libretexts
Find, Read, And Discover Energy Profile Diagram For Exothermic And Endothermic Reaction, Such Us:
- Exothermic Reactions Endothermic Reactions Energy Changes Examples Explained Compared Uses Importance Gcse Chemistry Revision Notes Ks4 Science Igcse O Level
- 2
- Chemistry Gcse Ks Learning
- Sage Books Secondary Science 11 To 16 A Practical Guide
- Enthalpy Change Of Reactions O Level Chemistry Notes
If you re searching for 2004 Honda Civic Fuse Box Diagram you've reached the right place. We ve got 104 graphics about 2004 honda civic fuse box diagram including images, photos, pictures, backgrounds, and more. In such page, we additionally provide number of graphics out there. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.

Ppt Exothermic And Endothermic Reactions Powerpoint Presentation Free Download Id 4499042 2004 Honda Civic Fuse Box Diagram
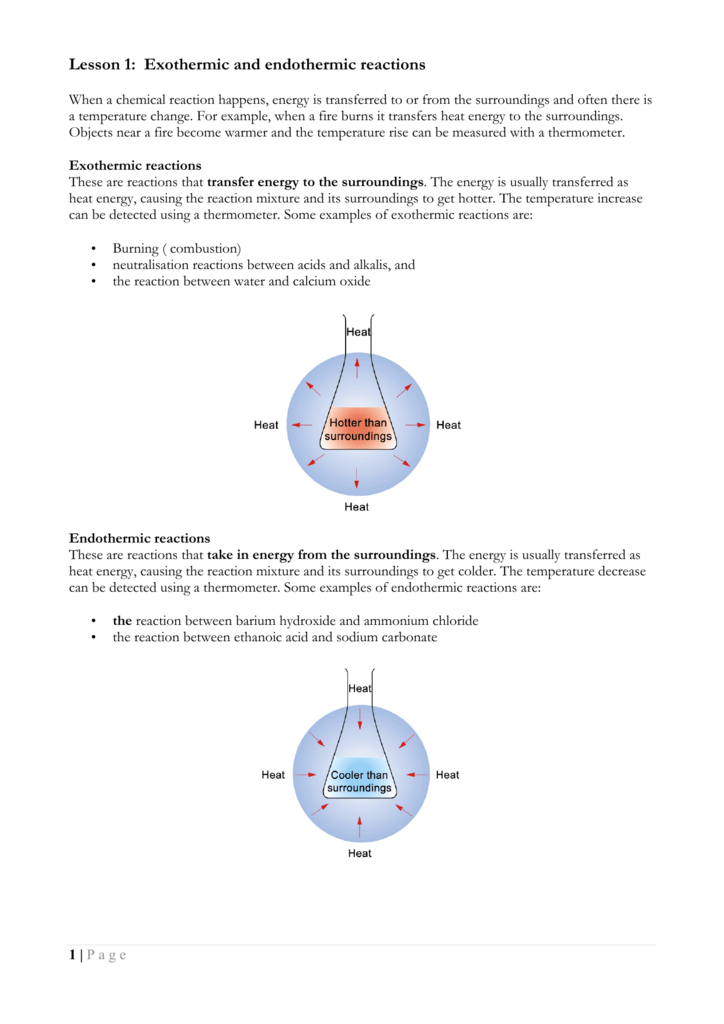
Exothermic reaction energy is released by the reaction.
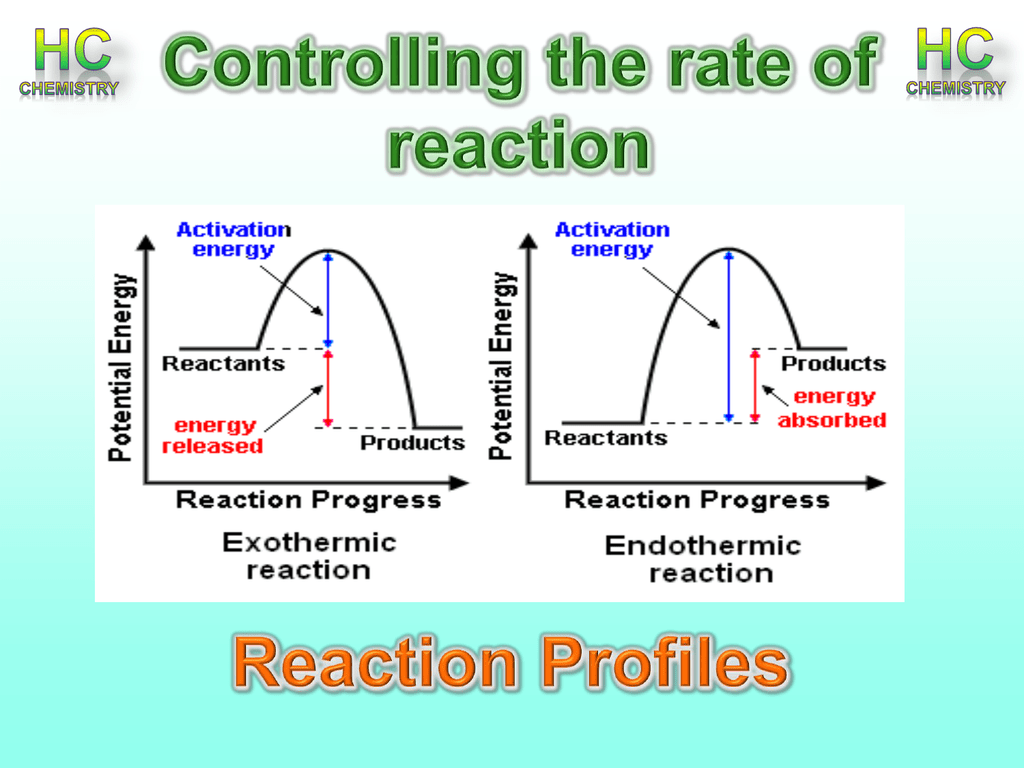
2004 honda civic fuse box diagram. Hesss law and reaction enthalpy change. This effect can be illustrated with an energy profile diagram. In the diagram above you can clearly see that you need an input of energy to get the reaction going.
On an energy profile the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products. This energy is given the symbol h and is different for different substances. This is represented on the reaction profile with a downwards arrow as the energy of the products is lower than the reactants.
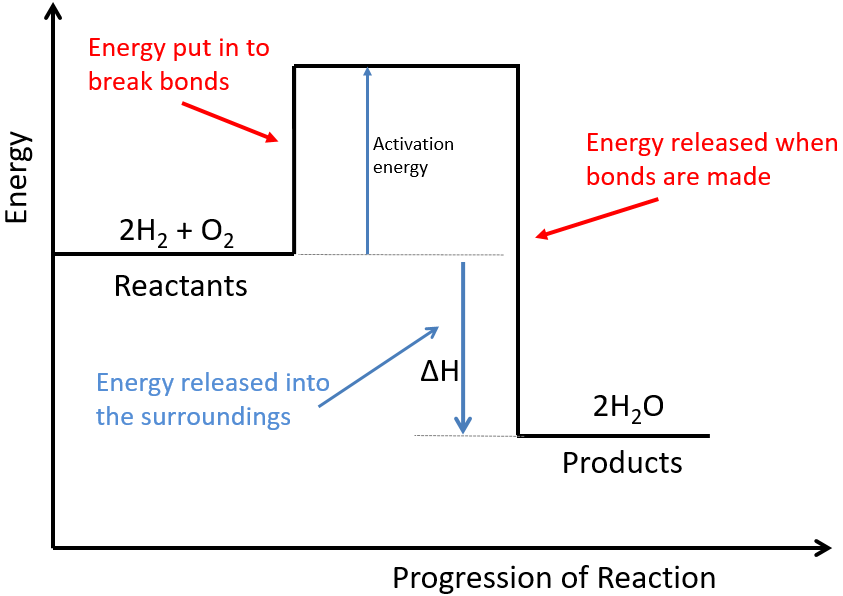
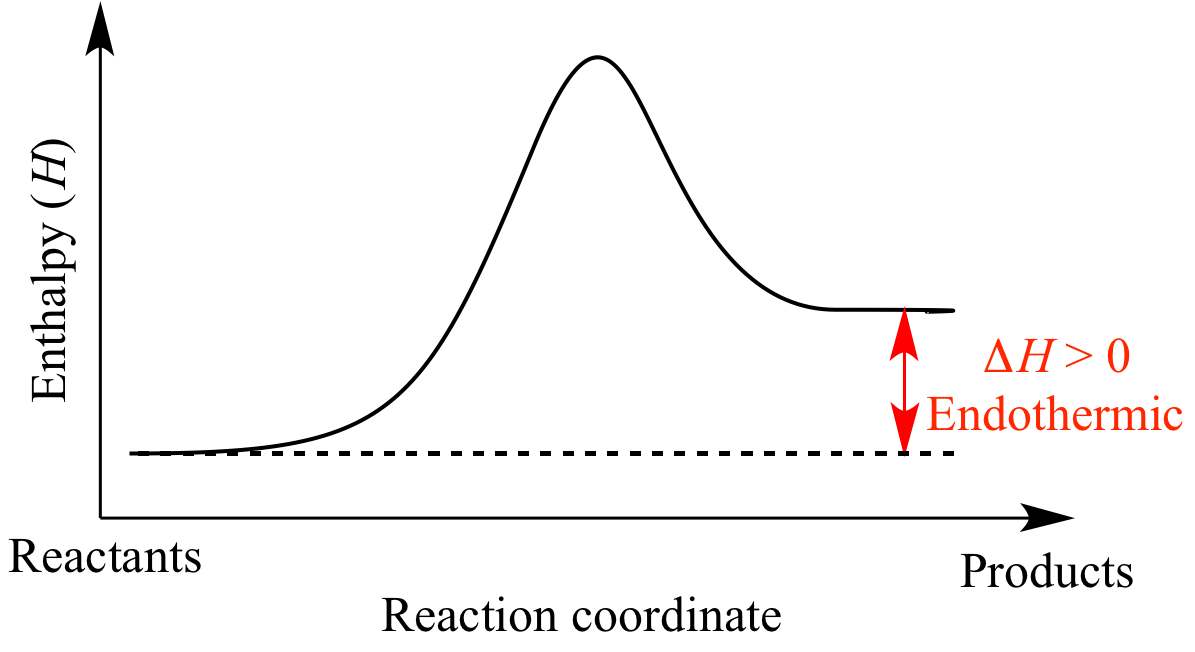
An exothermic reaction is one in which heat energy is given out. Below is a diagram showing the reaction profile for the thermal decomposition of calcium carbonate which is endothermic. The difference between these energy levels is dh.
Catalysis is the process of increasing the rate of a chemical reaction by adding a substance catalyzed reactions have a lower activation energy rate limiting free energy of activation than the corresponding uncatalyzed reaction resulting in a higher reaction. Gibbs free energy example. How does the energy level diagram show this reaction is exothermic.
If you had an endothermic reaction a simple energy profile for a non catalysed reaction would look like this. Gibbs free energy and spontaneity. Energy is given out in exothermic reactions.
Energy profile diagrams for endothermic and exothermic reactions every chemical substance has a certain amount of chemical energy. This is the currently selected item. Energy profiles for chemical reactions showing the activation energy.
It is difficult to measure the absolute energy of a substance but. For an exothermic reaction the products have less energy than the reactants. On an energy profile the energy of the reactants is lower than the energy of the products.
Once the activation energy barrier has been passed you can also see that you get even more energy released and so the reaction is overall exothermic. On an energy profile the energy of the reactants is greater than the energy of the products. More rigorous gibbs free energy spontaneity relationship.
Endothermic reaction energy is absorbed by the reaction. Energy profiles or energy diagrams for endothermic and exothermic reactions with or without a catalyst tutorial with worked examples for chemistry students. The activation energy is the minimum amount of energy required to start the reaction.
For an endothermic reaction the products have. The difference between the potential energies of products and reactants gives the heat of reaction. For an exothermic reaction more energy is released when bonds are formed than taken in when bonds are broken.
Unfortunately for many reactions the real shapes of the energy profiles are slightly different from these and the rest of this. An energy level diagram shows whether a reaction is exothermic or endothermic. The red arrow and blue arrows represent the exothermic and endothermic energy changes.
2004 Honda Civic Fuse Box Diagram, Endothermic Versus Exothermic Reactions
- Exothermic Reactions Endothermic Reactions Energy Changes Examples Explained Compared Uses Importance Gcse Chemistry Revision Notes Ks4 Science Igcse O Level
- Energy Changes
- Chapter 19 Chemical Kinetics
2004 Honda Civic Fuse Box Diagram, Chapter 19 Chemical Kinetics
- Steemitschool Exothermic And Endothermic Reactions Energy Profiles And Bond Energies Steemit
- Chemical Reactions And Energy Ck 12 Foundation
- 4 14 Represent Exothermic And Endothermic Reactions On A Simple Energy Level Diagram Igcse Chemistry Revision Help
2004 Honda Civic Fuse Box Diagram, 18 4 Potential Energy Diagrams Chemistry Libretexts
- Reaction Profiles Conjunto De Diapositivas
- Energy Profile Diagrams A Chemistry
- Endothermic Vs Exothermic Reactions Article Khan Academy
More From 2004 Honda Civic Fuse Box Diagram
- The Water Cycle Diagram For Kids
- 2005 Honda Pilot Fuse Box Diagram
- Endothermic Energy Diagram
- Unlabeled Picture Of The Heart
- Golgi Apparatus Diagram Labeled
Incoming Search Terms:
- How Can I Draw A Simple Energy Profile For An Exothermic Reaction In Which 100 Kj Mol 1 Is Evolved And Which Has An Activation Energy Of 50 Kjmol 1 Socratic Golgi Apparatus Diagram Labeled,
- Reaction Profiles Conjunto De Diapositivas Golgi Apparatus Diagram Labeled,
- Gradegorilla Chemistry Golgi Apparatus Diagram Labeled,
- What Is Difference Between Endothermic And Exothermic Reaction If Both Require Activation Energy Quora Golgi Apparatus Diagram Labeled,
- Energy Thermochemical Equations Golgi Apparatus Diagram Labeled,
- Igcse Chemistry 2017 3 5c Draw And Explain Energy Level Diagrams To Represent Exothermic And Endothermic Reactions Golgi Apparatus Diagram Labeled,