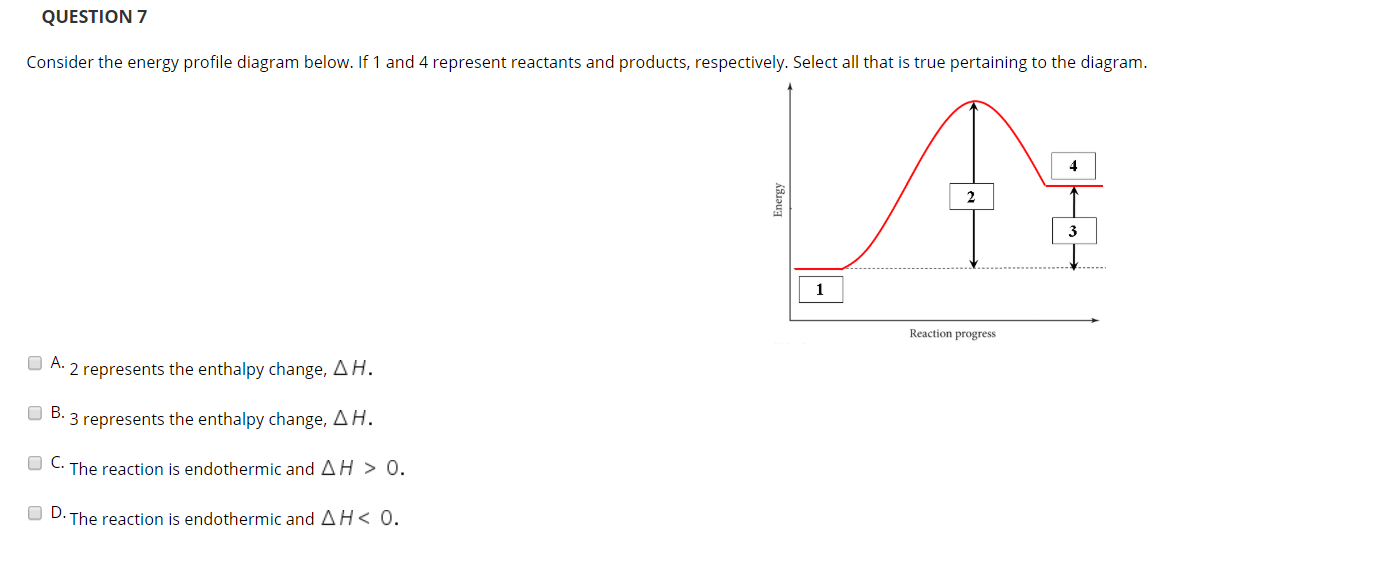
Energy Profile Diagram Endothermic, Enthalpy Change Of Reactions O Level Chemistry Notes
- Solved Each Of The Statements Given Below Is False Explain Wh Chegg Com
- Reaction Energy Profiles Activation Energy Exothermic Endothermic Reactions Catalysed Uncatalysed Reactions Gcse Notes Ks4 Science Igcse O Level Chemistry Revision
- Reaction Energy Profiles Activation Energy Exothermic Endothermic Reactions Catalysed Uncatalysed Reactions Gcse Notes Ks4 Science Igcse O Level Chemistry Revision
- Energetics 4 16 Enthalpy Changes
- Energy Changes
- Energy Profile Diagrams Endothermic Exothermic Reactions 1 Exothermic Reaction Chemical Energy Energy Level
- 4 14 Represent Exothermic And Endothermic Reactions On A Simple Energy Level Diagram Igcse2016
- Https Www Immanuelcollege Net Wp Content Uploads 2018 06 Y10 Combined Science Trilogy Energy Changes Higher Pdf
- Sage Books Secondary Science 11 To 16 A Practical Guide
- Sage Books Secondary Science 11 To 16 A Practical Guide
Find, Read, And Discover Energy Profile Diagram Endothermic, Such Us:
- Energy Profiles
- Energy Changes
- Endothermic Vs Exothermic Reactions Article Khan Academy
- Energy Energy Profile Of Endothermic Reactions
- Chemical Reaction Encyclopedia Article Citizendium
If you re looking for G59 Relay Wiring Diagram you've come to the perfect place. We have 104 graphics about g59 relay wiring diagram adding images, photos, pictures, wallpapers, and more. In such webpage, we additionally provide number of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
Energy diagram of one step exothermic reaction the vertical axis in this graph represents the potential energy.
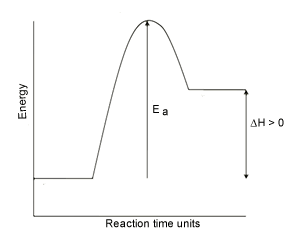
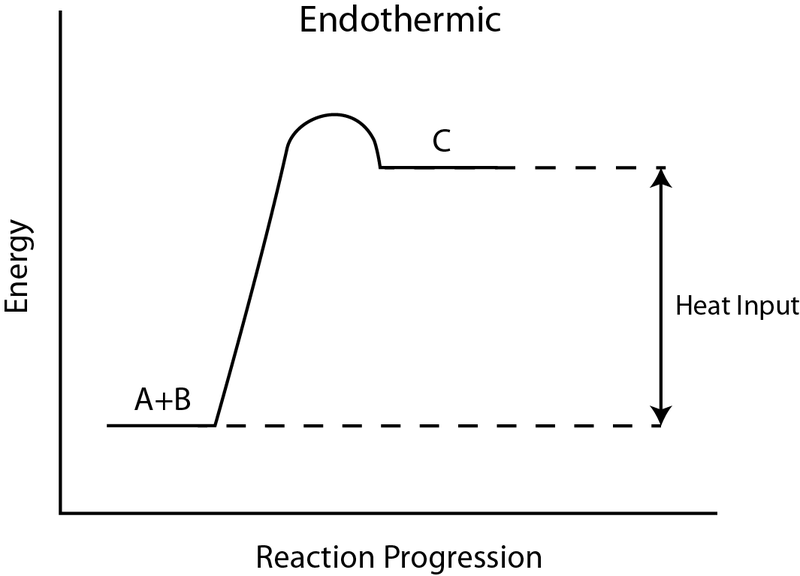
G59 relay wiring diagram. An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. In an endothermic reaction the reactants absorb heat energy from the surroundings to form products.
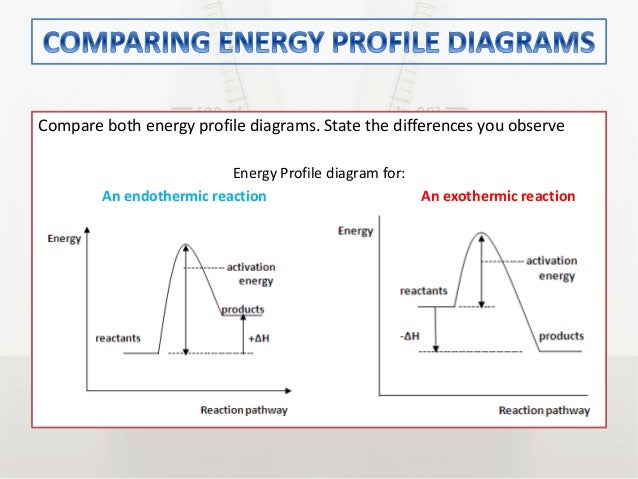
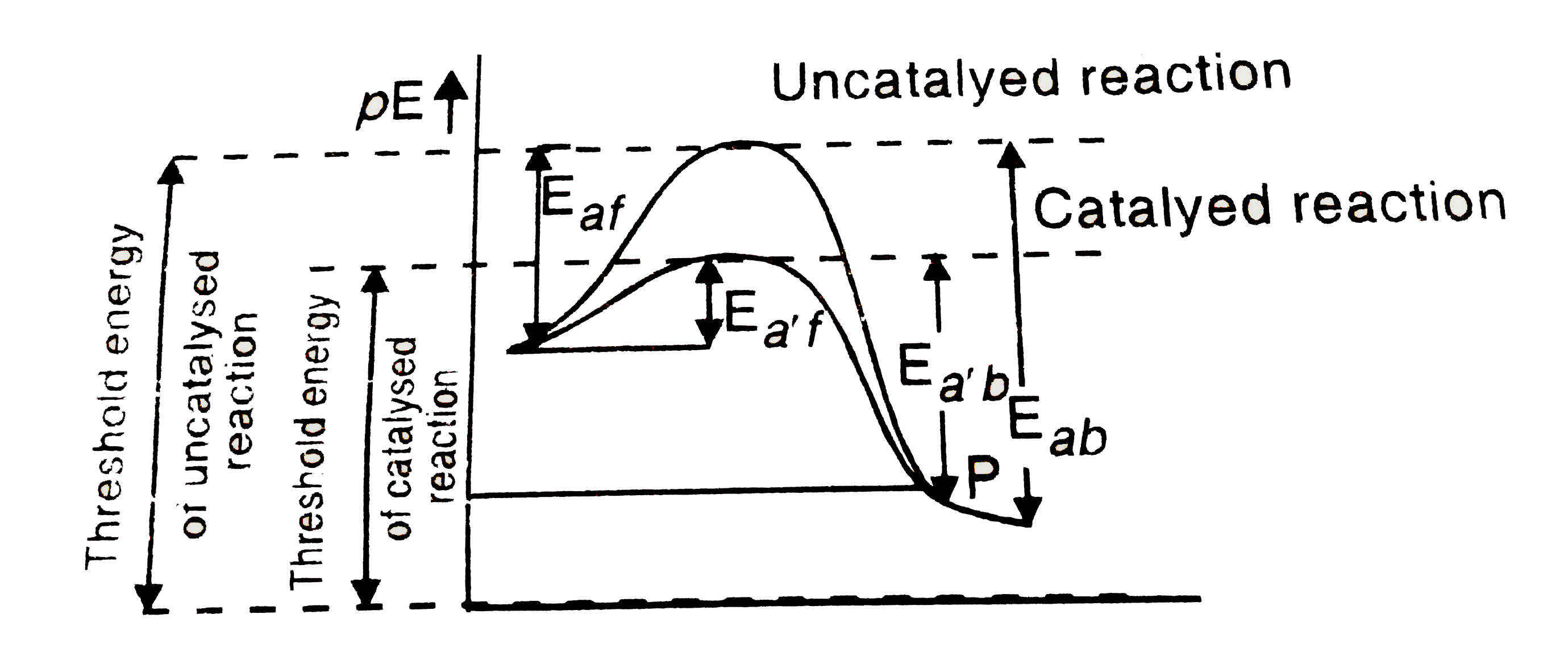
So an energy profile diagram shows the activation energy required and the enthalpy change for a reaction. Energy profile of endothermic reactions. The energy profile diagram for an exothermic reaction would be.
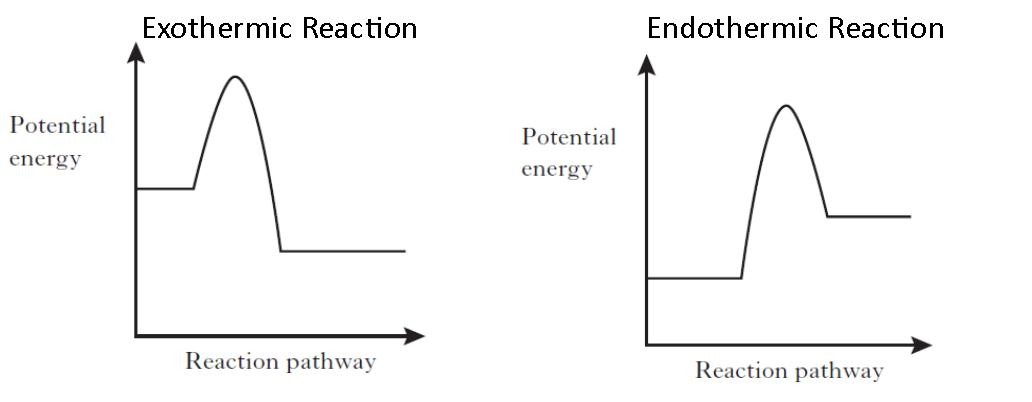
An energy level diagram shows whether a reaction is exothermic or endothermic. The figure below shows basic potential energy diagrams for an endothermic a and an exothermic b reaction. The energy profile diagram for an endothermic reaction would be.
On an energy profile the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. Posted on april 4 2019 by admin.
This diagram shows that overall the reaction is exothermic. What is an energy profile. If you have done any work involving activation energy or catalysis you will have come across diagrams like this.
The products have a lower energy than the reactants and so energy is released when the reaction happens. Important questions and answers energetics of chemical reactions. Enthalpy change dh is the amount of energy absorbed or released by a chemical reaction.
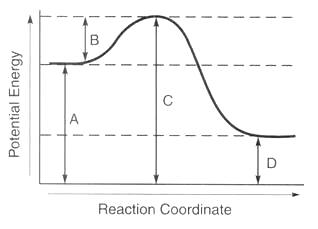
Therefore dh is positive. It shows the energy in the reactants and products and the difference in energy between them. Types of energy profile.
The transition state is the highest point on the graph and the activation energy is the energy difference between the reactants and the transition state. It shows the energy in the reactants and products and the difference in energy between them. Reaction energy profiles activation exothermic endothermic.
Thus the products formed have more energy than the reactans h products h reactants. The energy difference between the products and reactants represents the enthalpy change of the reaction. Endothermic reaction energy profile diagram.
Exothermic reactions when the activation energy is less than the energy released when the new bonds form there is an overall release of energy usually as heat released to the surroundings dh is negative and an exothermic reaction has taken place. Recall that the enthalpy change left delta h right is positive for an endothermic reaction and negative for.
G59 Relay Wiring Diagram, Why Is Free Radical Chlorination Exothermic Whereas Bromination Is Endothermic Chemistry Stack Exchange
- Rates And Kinematics Sciency
- Energy Diagram Practice
- Steemitschool Exothermic And Endothermic Reactions Energy Profiles And Bond Energies Steemit
G59 Relay Wiring Diagram, Chemistry M4 Energetics
- Gcse Chemistry What Are Energy Level Diagrams What Is The Energy Level Diagram For An Exothermic Reaction Gcse Science
- Exothermic And Endothermic Reactions Energy And Chemical Change Siyavula
- Energy Profile Diagram Lectures Notes Site
G59 Relay Wiring Diagram, Rmcstudy
- Exothermic Reaction Wikipedia
- Sage Books Secondary Science 11 To 16 A Practical Guide
- Rmcstudy
More From G59 Relay Wiring Diagram
- 2008 Ford Focus Fuse Box Diagram
- Eulerape
- Argon Atom Diagram
- 2010 Ford Mustang Fuse Box Diagram
- Plot Climax Chart
Incoming Search Terms:
- Reaction Profile Diagrams Youtube Plot Climax Chart,
- Energy Profile Diagram Lectures Notes Site Plot Climax Chart,
- Reaction Progress Diagrams Plot Climax Chart,
- Exothermic Endothermic Chemical Change Energy Foundations For High School Chemistry Plot Climax Chart,
- Igcse Chemistry 2017 3 5c Draw And Explain Energy Level Diagrams To Represent Exothermic And Endothermic Reactions Plot Climax Chart,
- Revision Chemical Change Mindset Chemical Change In The Following Energy Profile Diagram 3 1 Is The Breakdown Of Glucose An Endothermic Or An Exothermic Plot Climax Chart,