Energy Level Diagram Hydrogen, Draw A Neat Labelled Energy Level Diagram Of The Hydrogen Class 12 Physics Cbse
- Bohr S Theory Of The Hydrogen Atom Introduction To Physics Openstax Cnx
- Chem 101 The Quantum Mechanical Atom
- Hydrogen Emission Spectrum Spectroscopy Successive Ionisation Energy Patterns Related To Sub Shells And Group Of Periodic Table Gce A Level Revision Notes
- File Energy Level Diagrams For Hydrogen Png Wikimedia Commons
- Bohr S Theory Of The Hydrogen Atom Physics
- Bohr S Model Of Hydrogen Article Khan Academy
- Hydrogen Molecule Energy Level Diagram Big Chemical Encyclopedia
- Diagram Of Energy Levels Of Hydrogen Lines Physics Forums
- Spectral Lines
- A Simplified Energy Level Diagram Of Negative Hydrogen And Negative Download Scientific Diagram
Find, Read, And Discover Energy Level Diagram Hydrogen, Such Us:
- Diagram Of Energy Levels Of Hydrogen Lines Physics Forums
- Hydrogen Energies And Spectrum
- The Figure Shows An Energy Level Diagram For The Hydrogen Atom Se
- Chemistry Upper Secondary Ydp Animation Energy Level Diagram For The Hydrogen Atom
- Chapter 5
If you re looking for 2003 Honda Element Fuse Box Diagram you've arrived at the ideal place. We ve got 104 graphics about 2003 honda element fuse box diagram including pictures, pictures, photos, wallpapers, and much more. In these webpage, we also provide variety of images available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.

Draw To Scale An Energy Level Diagram For Hydrogen For Levels With N 1 2 3 4 5 6 Show Label The Following Transitions On The Diagram And Calculate The Wavelengths A The Transition Associated Wit Study Com 2003 Honda Element Fuse Box Diagram
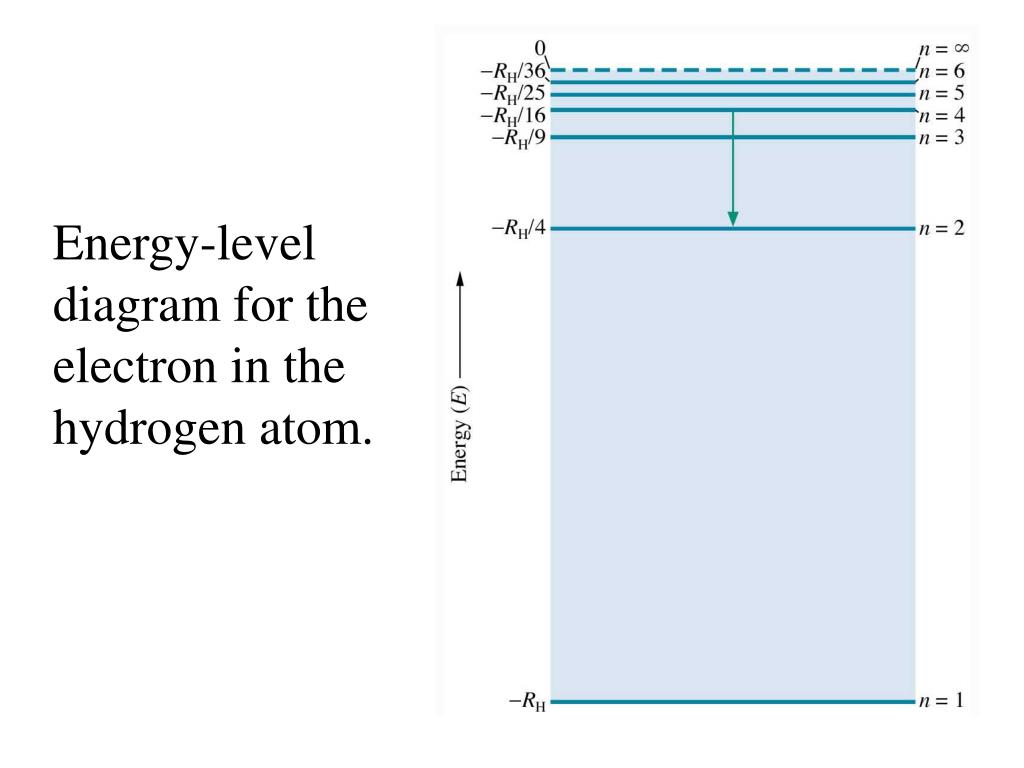
The ionization energy of an atom is the energy required to remove the electron completely from the atomtransition from ground state n 0 to infinity n.
2003 honda element fuse box diagram. Energy associated with the first orbit of the hydrogen atom is e 1 136 1 2 136 ev. 2 partial energy level diagram for hydrogen warning because of the draw a partial energy level diagram for hydrogen. At left is a hydrogen spectral tube excited by a 5000 volt transformer.
Greater value of bond order for h 2 molecule than h 2 ion shows that two h 2 molecule is more stable than h 2. Bond order value of 1 means that two hydrogen atoms are connected by a single bond. Violet 380 435nm blue435 500 nm cyan 500 520 nm green 520 565 nm yellow 565 590 nm orange 590 625 nm.
E 136 ev 1n f2 1n i2 atoms can also absorb photons. He then mathematically showed which energy level transitions correspond to the spectral lines in the atomic emission spectrum see below. If a photon with an energy equal to the energy difference between two levels is incident on an atom the photon can be absorbed raising the electron up to the higher level.
The electron energy level diagram for the hydrogen atom. The value 109677 cm 1 is known as rydberg constant for hydrogen. A an electron is incident on a hydrogen atom.
Partial energy level diagram for hydrogen. Emission spectrum and atomic spectra. Energy level diagram.
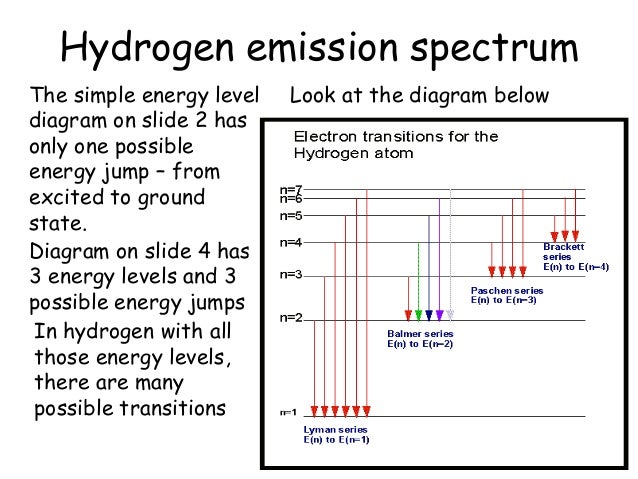
The atom then emits a photon of a characteristic frequency. He found that the four visible spectral lines corresponded to transitions from higher energy levels down to the second energy level left n2 right. N wave number of electromagnetic radiation.
The general formula for the hydrogen emission spectrum is given by. 41126 43477 48710 65842. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another.
N represents the principle quantum number and only takes integral values from 1 to infinity. Bond length of h 2 is smaller than that of h 2 ion. The ground state refers to the lowest energy level n1 in which the atom is the most stable.
The diagram below shows the lowest three energy levels of a hydrogen atom. It is called ground state energy of the hydrogen atom. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de excitation or by the wavelengths absorbed in an absorption.
Energy level diagram for hydrogen. As a result an electron in the ground state of the hydrogen atom is excited to the n 2 energy level. This is called the balmer series.
Energy level diagram for molecular orbitals. The three prominent hydrogen lines are shown at the right of the image through a 600 linesmm diffraction grating. In the hydrogen atom with z 1 the energy of the emitted photon can be found using.
Energy associated with the second orbit is given by e 2 136 2 2 34 ev. To learn more about hydrogen emission spectrum download byjus the learning app.
2003 Honda Element Fuse Box Diagram, Draw Energy Level Diagram For A Hydrogen Atom Showing The First Four Energy Levels Corresponding To N 1 2 3 And 4 Show Transitions Responsible For Physics Theory Shaalaa Com
- Ch 30 Nature Of Theatom
- For This Question Consider The Figure That Clutch Prep
- 6 2 The Bohr Model Chemistry
2003 Honda Element Fuse Box Diagram, Draw To Scale An Energy Level Diagram For Hydrogen For Levels With N 1 2 3 4 5 6 Show Label The Following Transitions On The Diagram And Calculate The Wavelengths A The Transition Associated Wit Study Com
- The Figure Shows An Energy Level Diagram For The Hydrogen Atom Several Transitions Are Youtube
- 8 6 Atomic Spectra And X Rays Physics Libretexts
- Hydrogen Atom Energy Level Diagram Of Hydrogen Atom
2003 Honda Element Fuse Box Diagram, Https Ocw Mit Edu Courses Chemistry 5 111 Principles Of Chemical Science Fall 2008 Readings And Lecture Notes Lecnotes05 Pdf
- 6 2 The Bohr Model Chemistry
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrjaqjlb8lpy2zmnxb7djnungtx4z3mbsdjg7v7iofb5bmcmoq Usqp Cau
- Spectral Lines Of Hydrogen Chemistry For Non Majors
More From 2003 Honda Element Fuse Box Diagram
- Metal Detector Circuit Using Ic 555 Pdf
- Diagram Of Hydrogen Spectrum
- Brake Light Wiring Colors
- Plantuml Flow Diagram
- Cd4047 Inverter Circuit Pdf
Incoming Search Terms:
- Hydrogen Energies And Spectrum Cd4047 Inverter Circuit Pdf,
- Hydrogen Energies And Spectrum Cd4047 Inverter Circuit Pdf,
- Solved Using The Energy Level Diagram Of Hydrogen Atom F Chegg Com Cd4047 Inverter Circuit Pdf,
- 6 2 The Bohr Model Chemistry Cd4047 Inverter Circuit Pdf,
- Figure 2 From Modeling Bohr S Theory Of Hydrogen Atom For Physics And Chemistry Education And Computer Science Graduates Semantic Scholar Cd4047 Inverter Circuit Pdf,
- The Figure Shows Energy Level Diagram Of Hydrogen Atom Cbse Class 12 Learn Cbse Forum Cd4047 Inverter Circuit Pdf,