Energy Level Diagram For Sulfur, Solved 3 Complete The Energy Level Diagram For The Lowes Chegg Com
- Energy Level Alignment At Interfaces In Metal Halide Perovskite Solar Cells Wang 2018 Advanced Materials Interfaces Wiley Online Library
- Exercise 3 Determining The Gas Density In Planetary Nebulae
- Solved 5 B Draw The Molecular Orbital Energy Level Di Chegg Com
- Formation Energy Of The Sulfur Vacancy In Different Charge States As A Download Scientific Diagram
- Chem4kids Com Sulfur Orbital And Bonding Info
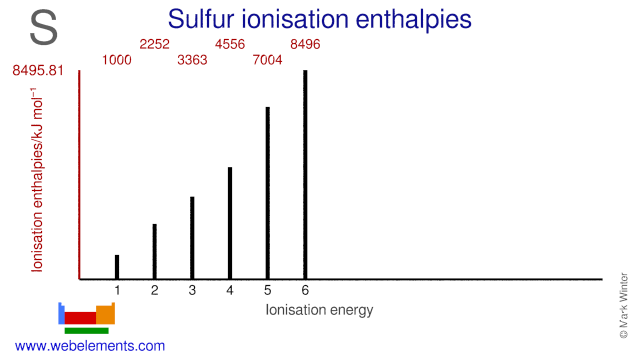
- Webelements Periodic Table Sulfur Properties Of Free Atoms
- Energy Level Diagram For The Molecular Orbitals Of H 2 O H And O Atom Download Scientific Diagram
- Https Zbiology Weebly Com Uploads 1 1 0 6 110667247 Unit 2 Practice Questions Pdf
- Structure Atom Hydrogen Carbon Nitrogen Oxigen Stock Vector Royalty Free 1124614544
- 1 Draw An Orbital Diagram For Sulfur Give A Full Set Of Four Quantum
Find, Read, And Discover Energy Level Diagram For Sulfur, Such Us:
- Ppt Electron Arrangement Powerpoint Presentation Free Download Id 6502162
- Energy Level Diagram For The Molecular Orbitals Of H 2 O H And O Atom Download Scientific Diagram
- Structure Of An Atom Hydrogen Carbon Nitrogen Oxigen Phosphorus Stock Photo Picture And Royalty Free Image Image 109250793
- What Is The Electron Configuration For S 2 Ion Socratic
- How To Find Out Unpaired Electron In S2 Molecule Chemistry Stack Exchange
If you are looking for 2010 Fuse Box Diagram you've arrived at the ideal place. We ve got 104 graphics about 2010 fuse box diagram including pictures, pictures, photos, wallpapers, and much more. In these web page, we additionally provide number of graphics available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gctmnhza Pfnw3rl9ifvtfqzjoxjhg05nielqw Usqp Cau 2010 Fuse Box Diagram
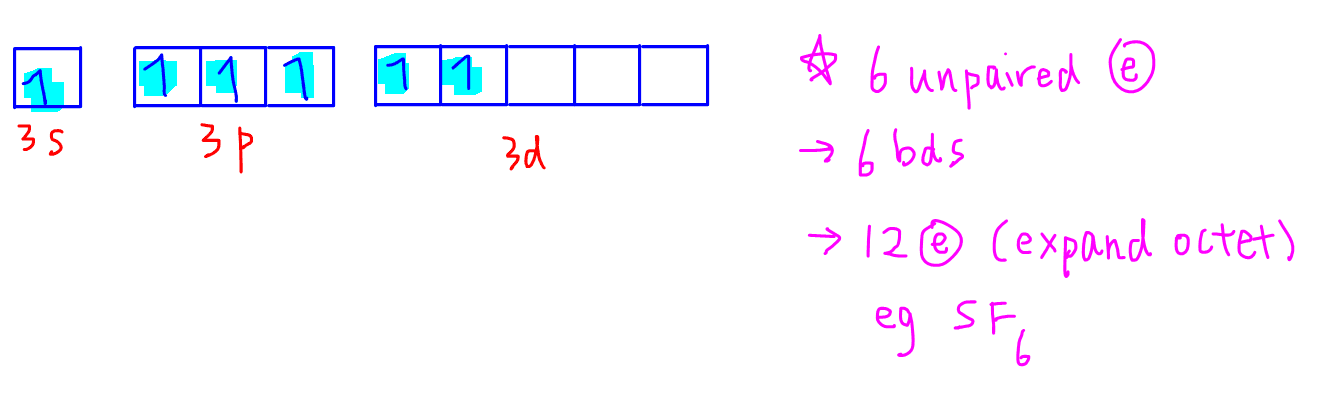
The boxes represent sulfurs orbitals.

2010 fuse box diagram. 1128 0c 38595 k 23504001 0f boiling point. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom there are 16 electrons. 1s2 2s2 2p6 3s2 3p4 if you need to fill in the little boxes heres one for you.
Chemistry from university of kentucky. A bohr diagram is a simplified visual representation of an atom that was developed by danish physicist niels bohr in 1913. Sulfur s energy levels of neutral sulfur s i configuration.
Therefore combining capacity of sulfur or valency is 2. Molecular orbital energy level diagram bond order and number of unpaired electrons. Each arrow represents one electron.
Sulfur uses its third shell as a valence shell. Bohr model of sulfur. 4446 0c 71775 k 83228 0f number of protonselectrons.
Physics suggests that electrons do not. Electronic configuration of sulfur is 286 as its atomic number is 16. Compunds ofsulphur like so3 so2 etc have a valency of 6.
Bohr diagram for sulfur. The electron dot diagram for a lone uncharged sulfur particle is an s with 6 electrons arranged around it 2 orbitals with 2 electrons and 2 orbitals with 1. 3s 2 3p 4.
Write the valence electron configuration of sulfur and determine the type of molecular orbitals formed in s 2. Bohr diagram for sulfur. The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each.
Home blog bohr diagram for sulfur november 4 2020. The orbital diagram is used to show the placement of electrons and correct usage of pairing and spin assignments to. Predict the relative energies of the molecular orbitals based on how close in energy the valence atomic orbitals are to one another.
The sulfur atom has 16 protons 16 neutrons and 16 electrons in three different energy levels or orbits. The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. 2 8 6 orbitals.
2010 Fuse Box Diagram, Electron Arrangement In Atoms Ck 12 Foundation
- Modified Bohr Diagram For An Atom Of Sulfur 34 Did I Do It Correctly Brainly In
- 0620 S16 Qp 41
- Why Do Compounds Like Sf6 And Sf4 Exist But Sh6 And Sh4 Don T Chemistry Stack Exchange
2010 Fuse Box Diagram, 5 P Problems Chemistry Libretexts
- Electronic Configuration The Atom Siyavula
- Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
- 8 2 Hybrid Atomic Orbitals Chemistry
2010 Fuse Box Diagram, Association Of Sulfur Amino Acid Consumption With Cardiometabolic Risk Factors Cross Sectional Findings From Nhanes Iii Eclinicalmedicine
- How To Find Out Unpaired Electron In S2 Molecule Chemistry Stack Exchange
- 3 5 Atomic Structure And The Periodic Table Creating
- 0620 S16 Qp 41
More From 2010 Fuse Box Diagram
- Platinum Bohr Diagram
- Creating Flow Charts In Excel
- Human Aorta Diagram
- Block Diagram Meaning
- Ethernet Wiring Diagram T568b
Incoming Search Terms:
- 10 5 Molecular Orbital Theory Chemistry Libretexts Ethernet Wiring Diagram T568b,
- 3 5 Atomic Structure And The Periodic Table Creating Ethernet Wiring Diagram T568b,
- Hydrogen Emission Spectrum Spectroscopy Successive Ionisation Energy Patterns Related To Sub Shells And Group Of Periodic Table Gce A Level Revision Notes Ethernet Wiring Diagram T568b,
- 8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts Ethernet Wiring Diagram T568b,
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gctmnhza Pfnw3rl9ifvtfqzjoxjhg05nielqw Usqp Cau Ethernet Wiring Diagram T568b,
- Figure 5 From Synthesis And Photoluminescence Of Zns Cu Nanoparticles Semantic Scholar Ethernet Wiring Diagram T568b,