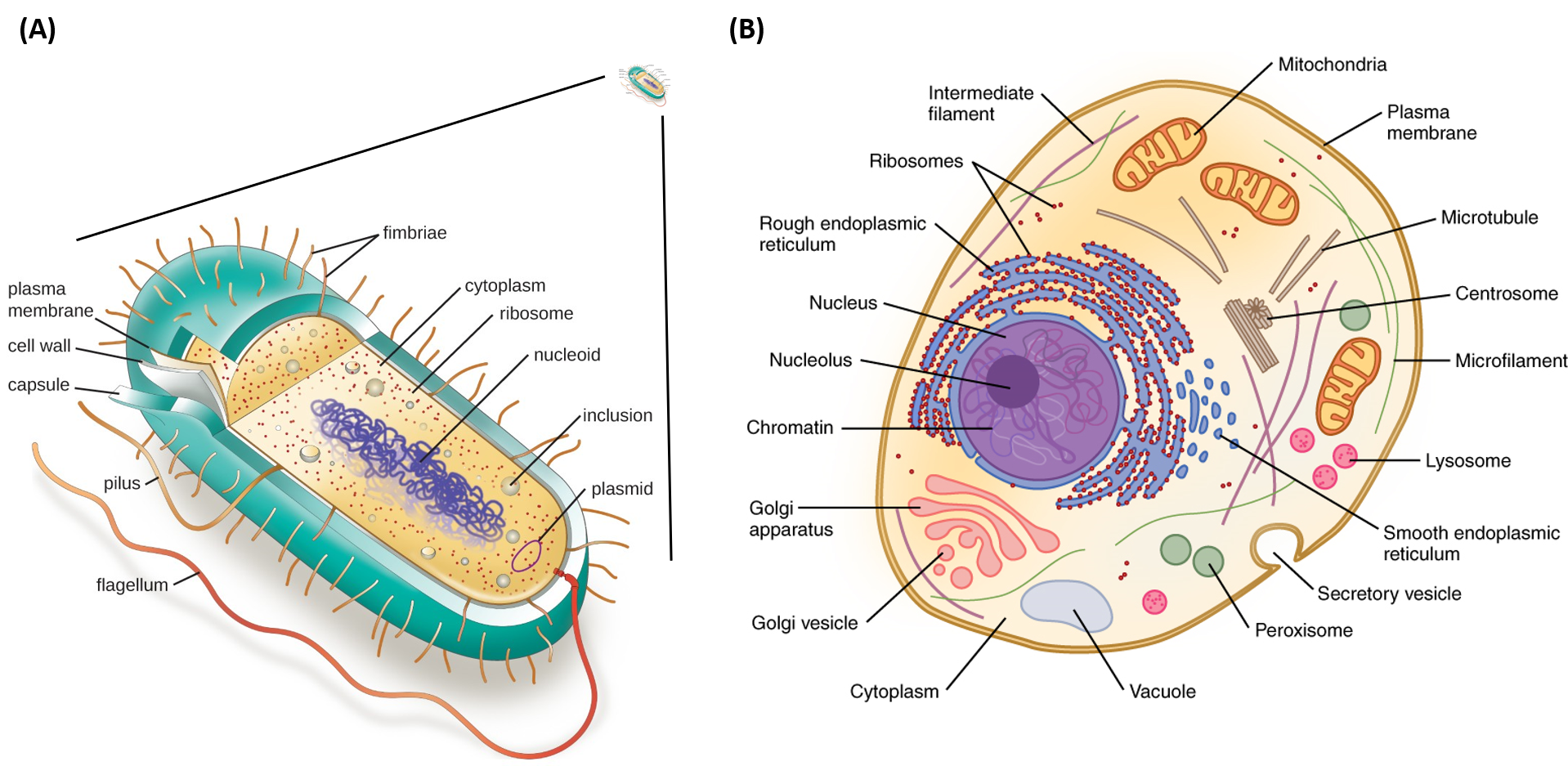
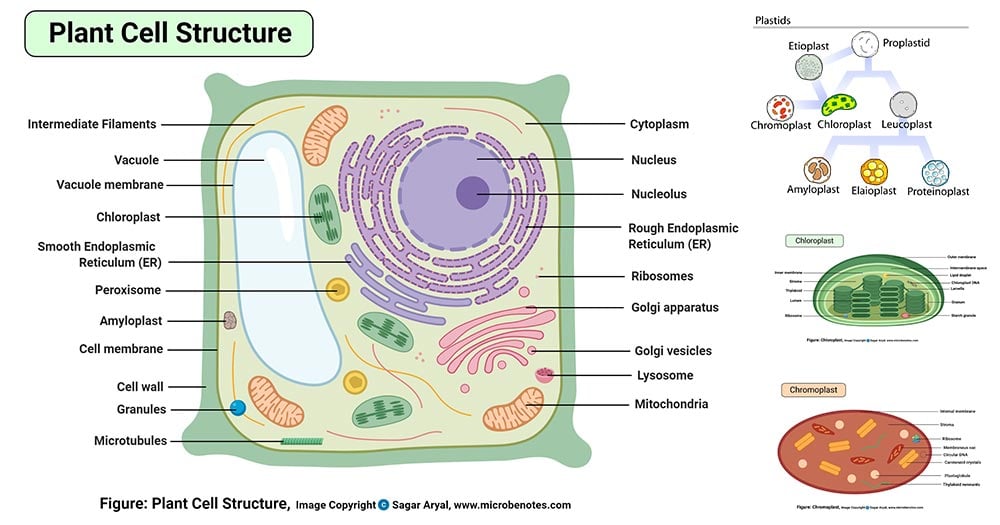
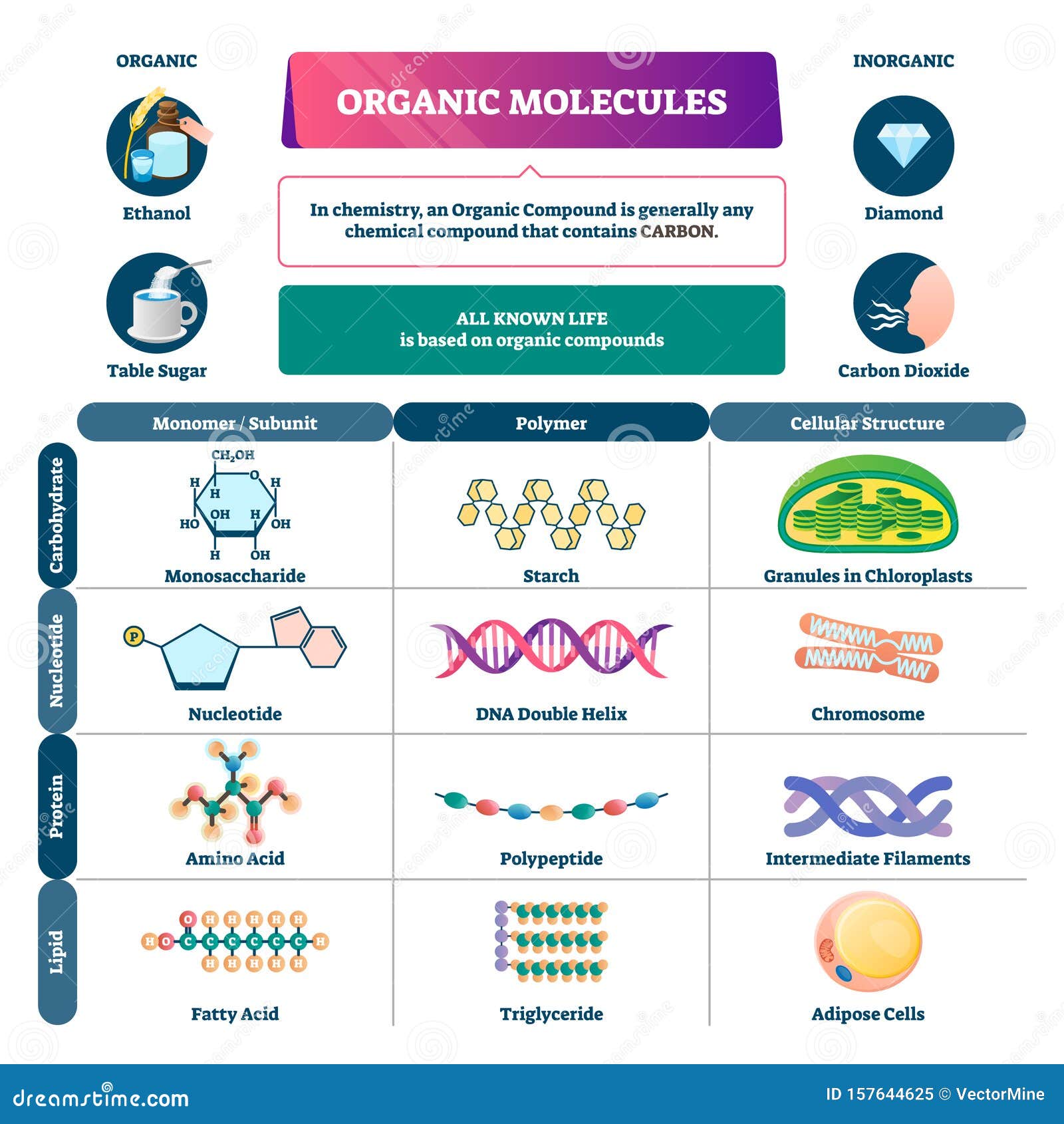
Cell Diagram Labeled Chemistry, Plant Cell Definition Labeled Diagram Structure Parts Organelles
- Lattice Structures In Crystalline Solids Chemistry
- Labeled Diagram Of Nerve Cell Biology Topperlearning Com Wwudaicc
- 20 3 Voltaic Cells Chemistry Libretexts
- Generalized Plant Cell
- A Well Labelled Diagram Of Animal Cell With Explanation
- Smartlearner
- Animal Cell Parts And Functions Withcarbon
- Labeled Animal Cell Doodle In 2020 Animal Cell Natural Form Art Biology Notes
- Galvanic Cells Galvanic Cells Sparknotes
- Cell Structure And Functions With Diagram
Find, Read, And Discover Cell Diagram Labeled Chemistry, Such Us:
- Chm 112 Lecture 29
- 17 3 Standard Reduction Potentials Chemistry
- Plant Cell Definition Labeled Diagram Structure Parts Organelles
- Drawing Labeling A Diagram Of A Electrochemical Cell Study Com
- Draw Neat And Labelled Diagram Of Dry Cell Chemistry Shaalaa Com
If you re looking for Heart Diagram Ks2 you've come to the perfect place. We have 104 images about heart diagram ks2 adding images, photos, pictures, backgrounds, and much more. In such page, we additionally provide number of graphics out there. Such as png, jpg, animated gifs, pic art, logo, black and white, transparent, etc.
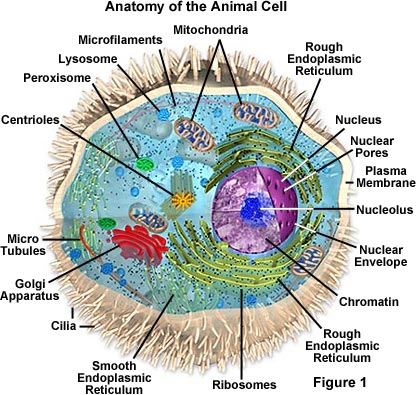
Just like different organs within the body plant cell structure includes various components known as cell organelles that perform different functions to sustain itself.
Heart diagram ks2. Nerve cells bone cells and liver cells for example all develop in ways that enable them to better perform their specific duties. Diagram and working of an electrolytic cell molten sodium chloride nacl can be subjected to electrolysis with the help of an electrolytic cell as illustrated below. The chemical reaction that occurs inside such cells is commonly referred to as electrolysis.
Electrolytic cells can be used to break down bauxite into aluminium and other components. A paste of hgo is used as an electrolyte. It is a rigid layer which is composed of cellulose glycoproteins lignin pectin and hemicellulose.
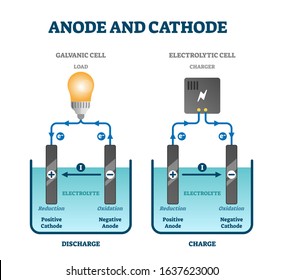
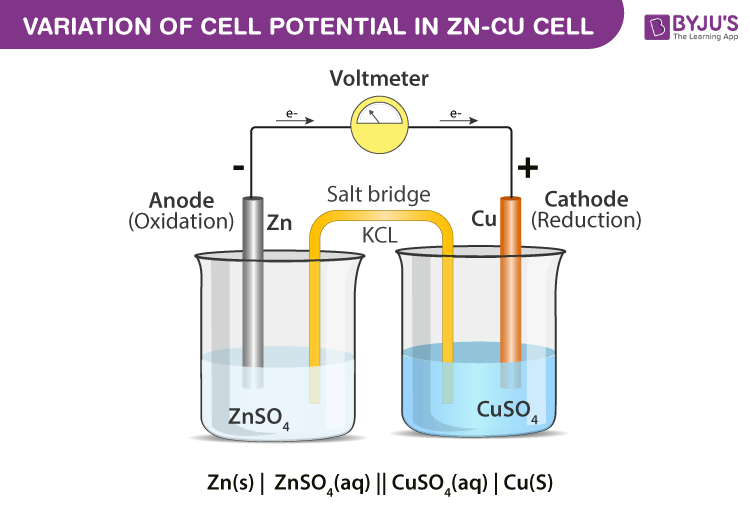
The zinc electrode is called the anode the electrode at which oxidation takes place and is labeled with a sign. This substantial difference in efficiency is because the. These cells are used only in devices that require a relatively low supply of electric current such as hearing aids and watches.
A block diagram of this fuel cell is provided below. Prokaryotic cells are not as complex as eukaryotic cellsthey have no true nucleus as the dna is not contained within a membrane or separated from the rest of the cell but is coiled up in a region of the cytoplasm called the nucleoid. The efficiency of the fuel cell described above in the generation of electricity generally approximates to 70 whereas thermal power plants have an efficiency of 40.
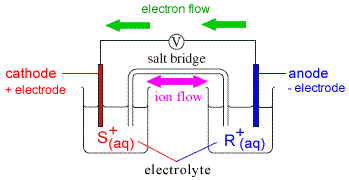
Another example of the primary cell is the mercury cell where a zinc mercury amalgam is used as an anode and carbon is used as a cathode. This cell will produce a little over one volt. For example a solid electrode in contact with a solution double vertical lines indicate the connection between the two electrolyte solutions such as a salt bridge.
Here two inert electrodes are dipped into molten sodium chloride which contains dissociated na cations and cl anions. The copper electrode is called the cathode the electrode at which reduction takes place and is labeled with a sign. The usual convention for writing a cell diagram is.
Bacterial cell anatomy and internal structure. Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. Prokaryotic organisms have varying cell shapes.
Constructing cell diagrams cell notation because it is somewhat cumbersome to describe any given galvanic cell in words a more convenient notation has been developed.
Heart Diagram Ks2, Cell Organelles Structure And Functions With Labeled Diagram
- Animal Cell The Definitive Guide Biology Dictionary
- Metabolic Labeling Strategy For Exosome Tracking Exosome Rna
- A General Strategy For Metabolic Labeling Of Plant Cell Wall Lignins Download Scientific Diagram
Heart Diagram Ks2, Cell Organelles Structure And Functions With Labeled Diagram
- Fuel Cells Batteries And Fuel Cells By Openstax Page 3 12 Jobilize
- 17 5 Batteries And Fuel Cells Chemistry
- Chm 112 Lecture 29
Heart Diagram Ks2, How Are The Chemical Reactions Of The Table Of Standard Reduction Potentials Experimentally Determined Chemistry Stack Exchange
- Describing Electrochemical Cells
- Fuel Cells Batteries And Fuel Cells By Openstax Page 3 12 Jobilize
- Organelle Membrane Specific Chemical Labeling And Dynamic Imaging In Living Cells Nature Chemical Biology
More From Heart Diagram Ks2
- Energy Flow Diagram Physics
- Ionic And Covalent Bonds Venn Diagram
- Saab 93 Fuse Box Diagram
- Honda Accord Radio Fuse
- 1998 Ford Ranger Fuse Box Diagram
Incoming Search Terms:
- Voltaic Cells 1998 Ford Ranger Fuse Box Diagram,
- Metabolic Labeling Strategy For Exosome Tracking Exosome Rna 1998 Ford Ranger Fuse Box Diagram,
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcsuqsccucug2xyrfvv37ucpra6vktjpe4w4lrcqzkdza9f2 Yml Usqp Cau 1998 Ford Ranger Fuse Box Diagram,
- Smartlearner 1998 Ford Ranger Fuse Box Diagram,
- How Are The Chemical Reactions Of The Table Of Standard Reduction Potentials Experimentally Determined Chemistry Stack Exchange 1998 Ford Ranger Fuse Box Diagram,
- Live Cell Imaging Of Bacterial Cells Pyrenoylpyrrole Based Fluorescence Labeling Chemical Biology Drug Design X Mol 1998 Ford Ranger Fuse Box Diagram,